Doris K. Wu, Ph.D., Chief
Research Statement
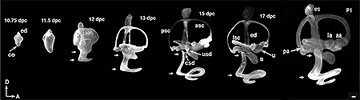
Morphogenesis of the mouse inner ear from embryonic day 10.75 to postnatal day 1. Mouse specimens from various ages were fixed, cleared, and the lumen of the inner ear was filled with a latex paint solution (Cantos et al. 2000; for a detailed description of this paint filling technique, see Morsli et al. 1998).
We rely on the inner ear, an intricate sensory organ, to hear and to maintain balance. Formation of this organ is a complex process that occurs in a precise temporal sequence16. This temporal sequence is largely initiated by cues from tissues surrounding the ear primordium, followed by a cascade of molecular events within the ear tissue. Any missteps in this process will most likely result in some degree of dysfunction affecting the abilities to hear and maintain balance.
The goal of the laboratory is to identify the molecular mechanisms underlying the formation of this complex structure. Using mouse, chicken, and zebrafish as animal models, we focus on identifying the external cues that instruct the ear rudiment about its positional information—for example, where to form the cochlea (hearing apparatus) versus the semi-circular canals (non-sensory vestibular structures)2–6,12,14,17. Another focus of the laboratory is to determine the cascades of events induced by external cues, which specify all aspects of inner ear development including the three primary cell types (neural, sensory, and nonsensory) of the ear rudiment2 and the identity of each sensory structure and the types of sensory hair cells and polarity within2,7–11,13,15. By understanding the normal development of the inner ear at a molecular level, we may help to design better strategies to alleviate hearing and balancing disorders.
Lab staff as of October 2018. Front row L-R: Tao Jiang, Ph.D., Loksum Wong, Ph.D., Sho Ota, Ph.D., Doris K. Wu, Ph.D. Back row L-R: Kazuya Ono, Ph.D., Yosuke Tona, Ph.D., Michael Mulheisen, biologist, Youngrae Ji, Ph.D.

