Why Changes to Clinical Trial Policies?
To enhance its stewardship of clinical trials, the National Institutes of Health (NIH) updated its clinical trial policies effective 2017-2018. See the diagram below for details. The updated policies are intended to improve efficiency, accountability, and transparency throughout the lifespan of grant applications and contract proposals, including:
- Approach to funding opportunities.
- Information collected in applications/proposals.
- Review criteria.
- Post-award management and oversight.
- Dissemination of research results.
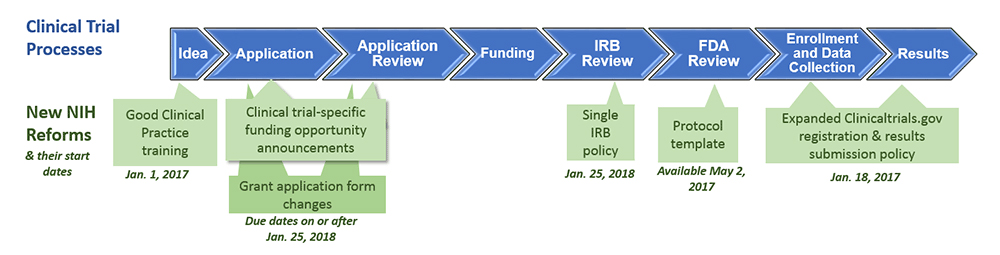
Good Clinical Practice Training
Effective January 1, 2017, NIH expects all NIH-funded clinical investigators and clinical trial staff who are involved in the design, conduct, oversight, or management of clinical trials to be trained in Good Clinical Practice (GCP).
Clinical Trial-Specific Funding Opportunities
Beginning with January 25, 2018 due dates, all applications proposing clinical trials must be submitted through a funding opportunity announcement (FOA) designated specifically for clinical trials.
New Human Subjects and Clinical Trial Information Form
A new Human Subjects and Clinical Trial Information form is required for all human subjects and/or clinical trial research beginning with January 25, 2018, due dates. The new form will be included in FORMS-F application packages.
Single IRB Policy for Multi-Site Research
For grant applications proposing the conduct of domestic multi-site clinical trials, the new policy requires the use of a single Institutional Review Board (IRB). This policy is effective for due dates January 25, 2018, and beyond, and for contract solicitations published on or after January 25, 2018.
Clinical Trials Protocol Template
If your application includes Phase II or III clinical trials that require Investigational New Drug application (IND) or Investigational Device Exemption (IDE) applications, an NIH-FDA template with instructional and sample text can help you write your protocols. This template is modifiable to any type of clinical trial, including clinical trials with behavioral interventions.
Clinicaltrials.gov Registration and Reporting
A new regulation and NIH policy has expanded Clinicaltrials.gov registration and reporting to all NIH-funded clinical trials.
More Information
For details on NIH clinical trial requirements, see the NIH Office of Extramural Research’s Clinical Trial Requirements for Grants and Contracts and a video, Overview of New NIH Policies on Human Subjects Research.

A still shot of a video, Overview of New NIH Policies on Human Subjects Research. Captions are provided for the video.

