- Download Congressional Justification 2023 (1.64MB PDF)
FY 2023 Budget
DEPARTMENT OF HEALTH AND HUMAN SERVICES
NATIONAL INSTITUTES OF HEALTH
National Institute on Deafness and Other Communication Disorders
On this page:
- Director’s Overview
- NIDCD Fact Sheet
- Major Changes in Budget Request
- Budget Mechanism Table
- Appropriations Language
- Summary of Changes
- Budget Graphs
- Organization Chart
- Budget Authority by Activity
- Justification of Budget Request
- Program Descriptions
- Appropriations History
- Authorizing Legislation
- Amounts Available for Obligation
- Budget Authority by Object Class
- Salaries and Expenses
- Detail of Full-Time Equivalent Employment (FTE)
- Detail of Positions
Director’s Overview

M.D., M.S., M.B.A.
Director, NIDCD
About 20 percent of the United States’ population have a hearing or other communication disorder.1 For these individuals, the basic components of communication—sensing, interpreting, and responding to people and things in our environment—can be challenging. The National Institute on Deafness and Other Communication Disorders (NIDCD) manages a broad portfolio of basic, translational, clinical, and public health research focused on human communication and associated disorders in three program areas: hearing and balance; taste and smell; and voice, speech, and language. NIDCD’s vision is to advance the science of communication to improve lives.
Changes in research to quickly respond to needs associated with the pandemic: NIDCD is actively pursuing new research to fill a knowledge gap on how coronavirus disease 2019 (COVID-19) impacts sensory and communication functions in hearing, balance, taste, smell, voice, speech, and language. Losing one’s sense of smell is a strong predictor of COVID-19 disease. Anosmia (lack of sense of smell), hyposmia (reduced sense of smell), and dysgeusia (change in taste) have been reported in 30-80 percent of cases of adults infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).2 NIDCD provided supplemental funds to 18 ongoing NIDCD grants to enable researchers to address the pathology, prevention, diagnosis, sequelae, or treatment of COVID-19. One study focuses on understanding how COVID-19 causes loss of smell and whether that loss of smell can be used as a valid early predictor of SARS-CoV-2 infection. Another study aims to identify the genetic basis for why some individuals lose smell when infected with SARS-CoV2 and others do not. Finally, a third study is examining the biological mechanism for persistent smell loss in COVID-19 long-haulers, as 5-10 percent of people have severe to complete smell loss six months after infection. Further, NIDCD is the lead on one of the National Institutes of Health’s (NIH) Rapid Acceleration of Diagnostics Radical (RADx-rad) initiatives to support development of quick and inexpensive tests to assess taste and smell function in symptomatic and asymptomatic individuals with COVID-19. Additional NIDCD awards are supporting community and digital healthcare interventions to address the social, behavioral, and economic health impacts of COVID-19 in vulnerable and health disparate populations.
NIDCD was also able to help ensure research support to early career scientists and scientists whose career trajectories were significantly impacted by the pandemic.3 NIDCD joined NIH in taking steps to allow flexibility and options so grantees could sustain their research and prioritize safety for themselves, their lab members, and research participants during the pandemic. Some of the actions undertaken by NIH and NIDCD include delaying the start dates of some awards to accommodate lab closures and permitting continued payment of salaries on research grants despite lab closures and increased clinical responsibilities during the pandemic. NIDCD also approved generous no-cost extensions to project end dates for the purpose of completing grant activities as well as modifications of existing grant protocols to allow telehealth/virtual intervention approaches. Further, grantees were encouraged to shift their research focus to other research or convert in-person activities to virtual platforms, when appropriate, to focus on safe conduct of research. NIDCD extended the eligibility period for certain types of grant mechanisms for some early career applicants, and we allowed beginning investigators to request grant extensions with additional funds to training grants and early career research awards. These actions help solidify the research enterprise and support the research pipeline so that scientists are positioned to return to full research capacity when the pandemic allows.
The necessity for considering health disparities in research and medicine: Disparity and inequity in healthcare, including access and outcomes, poses hardships for the American healthcare system. For decades, researchers have documented inequalities in health and healthcare in racial/ethnic minority populations, socioeconomically disadvantaged populations, sexual and gender minority populations, individuals with disabilities, and rural populations. Communication disorders are not immune to the known and unknown factors that contribute to these inequalities. Despite this, there has been a limited effort to date to systematically explore health disparities, including issues of racism and discrimination, in communication disorders among underrepresented groups or to resolve these disparities and promote equity in care. Sufficient evidence exists to affirm that underrepresented minorities’ communication disorders have been and continue to be understudied and underserved. NIDCD issued a Notice of Special Interest on Health Disparities and Inequities Research, which encourages grant applications that 1) advance the understanding or reduce the impact of health disparities and inequities in communication disorders among racial/ethnic minority and other underrepresented populations, and 2) address the influence or reduction of racism or discrimination in causing or sustaining health disparities and inequities.4
NIDCD recognizes that for the United States to remain a global leader in scientific discovery and innovation, it is dependent upon a pool of highly talented scientists from diverse backgrounds. NIDCD has issued two funding opportunity announcements (FOAs) to foster the development of researchers from diverse backgrounds and across many career stages. One FOA will support creative educational activities with a primary focus on mentoring activities and institutional programs aimed at improving the caliber of mentorship.5 The other FOA will support outstanding educational activities with a primary focus on research experiences.6 The proposed research experiences may be targeted to undergraduates, graduate students, post-doctorates, or early- to mid-career faculty and will be designed to extend those individuals’ research skills, experiences, and knowledge base. The purpose will be to foster their ability to transition to individual research awards.
New directions in clinical research with a focus on individuals with communication disorders: NIDCD has been able to expand support for innovative clinical trials that may one day lead to treatments that improve the quality of life for individuals with communication disorders.

San Francisco
Pilot Clinical Trial for Speech Prosthesis: Conditions such as stroke and neurodegenerative disease can cause anarthria—the loss of the ability to articulate speech. Anarthria hinders communication and reduces quality of life. Researchers have been working to develop brain-computer interfaces that help people with anarthria communicate. With a typical interface, the user must spell out messages one letter at a time. An interface that lets the user generate whole words at a time would allow much more efficient and natural communication. A team of NIDCD-funded researchers have developed a “speech neuroprosthesis.”7 This device directly decodes brain activity into words and sentences in real time. In an individual who was unable to speak for 16 years, the researchers implanted an array of electrodes into the part of the person’s brain implicated in speech processing. The researchers used machine learning to recognize patterns in the brain activity and associate these with what word the user was trying to say. While the vocabulary pool was limited to 50 words, the individual was able to say sentences while the device decoded his brain activity. Although this study only had one participant to date, the results are a significant advance. NIDCD provides funding to continue enrollment of more participants into this clinical trial with expansion of the word sets for all participants.8
Vestibular Prosthesis for Balance Disorders: Tens of thousands of people suffer from severe loss of balance caused by genetic defects, drug reactions, Ménière’s disease, viral infection or other diseases that impact the inner ear’s vestibular organs, which regulate balance. For about 1.8 million adults worldwide with bilateral vestibular hypofunction (BVH)—loss of the inner ears’ sense of balance—walking requires constant attention to avoid a fall. NIDCD is supporting a clinical trial that aims to treat BVH in adults through use of a vestibular prothesis—a miniature device that includes components worn outside the body as well as implanted into the ear that work together to help restore the sense of balance to affected individuals.9 Recently, researchers from this clinical trial showed that they could facilitate walking, relieve dizziness, and improve the quality of life in people with BVH who were surgically implanted with this prosthesis, which works by transmission of signals from the external component to the internally implanted component. This electrically bypasses malfunctioning areas of the inner ear and partially restores the sensation of balance.10 The clinical trial continues to recruit new participants; and, even after one year, individuals with the vestibular protheses have successfully returned to daily activities that would otherwise have been impossible without this intervention.
Improving Language and Cognition in Children with Down Syndrome: In 2018, NIH launched the INvestigation of Co-occurring conditions across the Lifespan to Understand Down syndromE, (INCLUDE) initiative to address critical health and quality-of-life needs for individuals with Down syndrome (DS). NIDCD participates in the INCLUDE initiative with the purpose of understanding the natural history of communication disorders and promote early identification and clinical management throughout the lifespan in individuals with DS. As a result of that participation, NIDCD is now funding a clinical trial in children and young adults with DS that have severe, obstructive sleep apnea.11 While continuous positive airway pressure (CPAP), or sleep masks, may be a common treatment option for obstructive sleep apnea, the majority of children with DS are noncompliant with CPAP due to co-occurring sensory conditions. In a Phase I clinical trial, investigators first looked at hypoglossal nerve stimulation (HGS), a therapy approved by the U.S. Food and Drug Administration (FDA) for moderate to severe obstructive sleep apnea in adults who failed or cannot tolerate CPAP treatment. HGS involves implanting a device that stimulates the nerve controlling tongue movement. The researchers’ first objective was to determine if HGS implantation was safe for the treatment of obstructive sleep apnea in children and young adults with DS. The secondary objectives were to identify changes in sleep quality and evaluate compliance. In addition, they found an interesting observation—parents reported their children were more attentive and doing better in school. Many parents also reported their children were speaking with more clarity. Next the scientists performed a pilot study in five children with DS. These children showed improvements in IQ scores, diversity of vocabulary and complexity in spoken language. Because of these improvements in articulation and language expression, the study has moved on as a Phase 2 multicenter trial. The results of this study are particularly meaningful because of the potential improvements in cognitive and expressive language outcomes. Researchers will track brief attention, sustained attention, processing speed, verbal intelligence, and executive functioning as well as measure the diversity of vocabulary and syntactic complexity. If the intervention trial is successful, it could mean immediate changes for how sleep apnea is managed among children with DS to improve quality of life throughout their lives.

Developing New Standardized Treatments for Children with Asymmetrical or Single Sided Hearing Loss: In another NIDCD-supported clinical trial, clinicians are surgically treating children with asymmetric hearing loss (AHL; defined as severe to profound hearing loss in one ear and mild to moderate hearing loss in the other ear) or single-sided deafness (SSD; defined as severe to profound hearing loss in one ear and normal hearing function in the other) with a cochlear implant.12 Since 2000, cochlear implants have been FDA-approved for use in eligible children beginning at 12 months of age. Children who receive a cochlear implant followed by intensive therapy before they are 18 months old typically are better able to hear and speak compared to their peers who receive implants when they are older. Many of these children also develop spoken language skills at a rate comparable to children with typical hearing and many succeed in mainstream classrooms. Recently, the FDA granted approval to implant a subset of children with AHL or SSD; however, the approval is restrictive as it is limited to children with profound hearing loss and very low word recognition in the poor ear. Therefore, this NIDCD longitudinal, prospective, multi-center clinical trial will help provide evidence for cochlear implant candidacy criteria, assessment tools, or performance outcomes over time for children with AHL or SSD. This evidence-based approach is integral to the establishment of standardized and equitable treatments for these children.
NIDCD Fact Sheet
Advancing the science of communication to improve lives

M.D., M.S., M.B.A.
Director, NIDCD
For over 30 years, NIDCD's focus has been to bring national attention to normal and disordered processes of hearing, balance, taste, smell, voice, speech, and language and to contribute to advances in biomedical and behavioral research that will improve the lives of the tens of millions of people with deafness and other communication disorders. NIDCD employs a wide range of research approaches such as molecular genetics, biomedical imaging, cellular biology, nanotechnology, psychoacoustics, and structural and functional biology to advance the development of better diagnostics, prevention strategies, and treatments.
Funding Level History
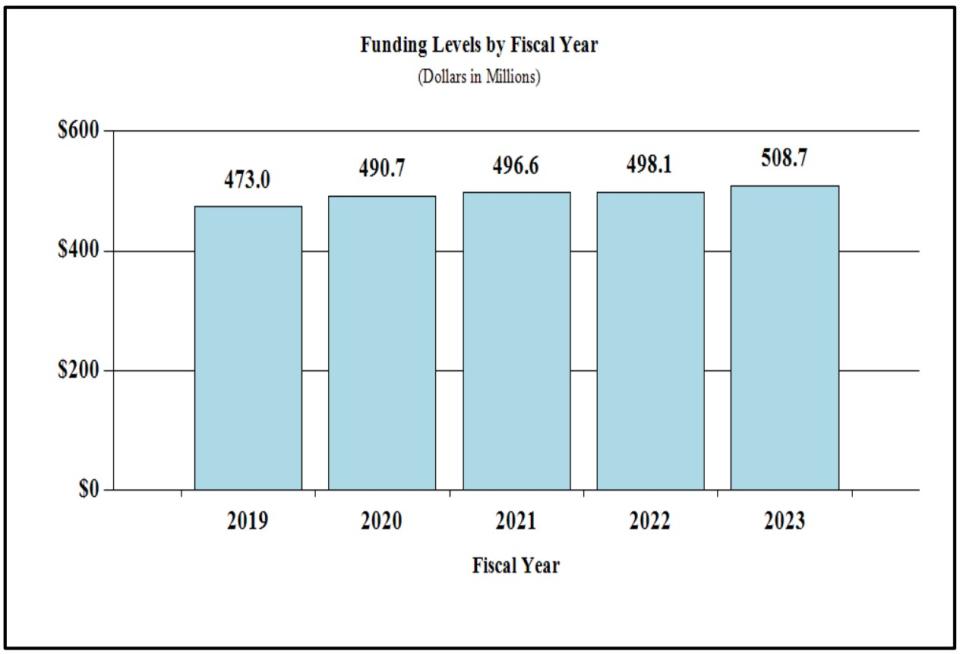
President's Budget 2023: $508,704,000
FY 2022 CR: $498,076,000
FY 2021: $496,578,000
FY 2020: $490,692,000
FY 2019: $472,996,000
Funding by Mission Area (FY 2021)
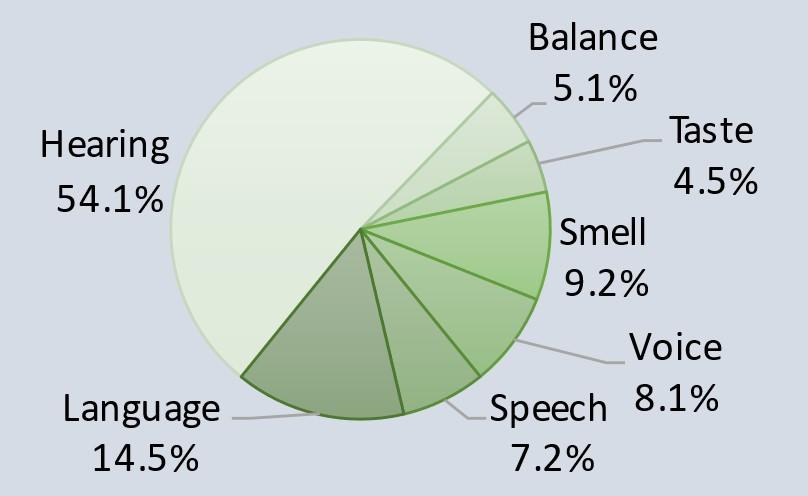
FY 2021 Facts & Figures
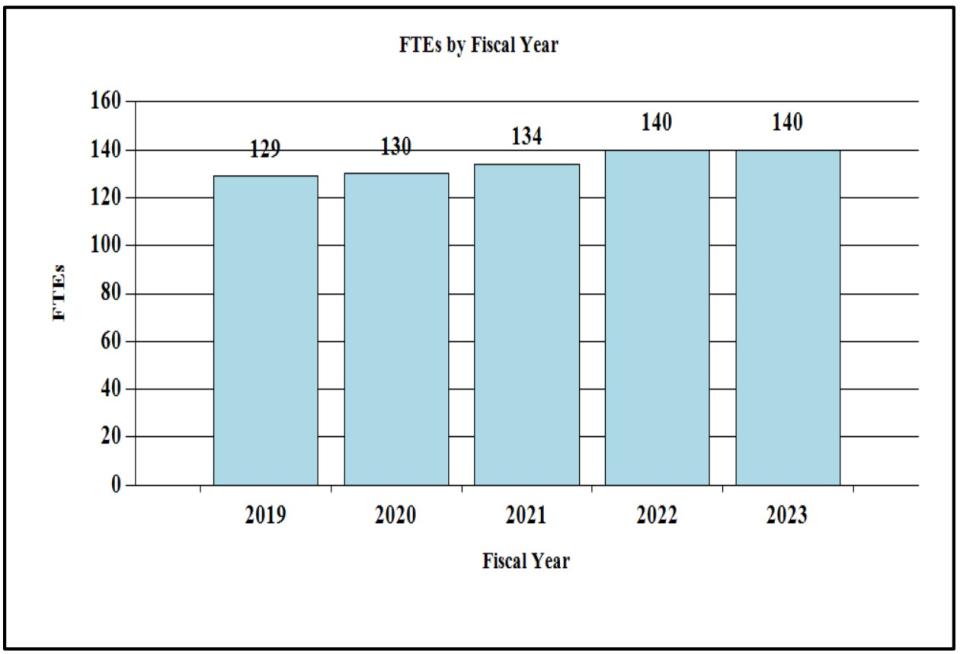
- 134 Full Time Equivalent Employees
- 210 Principal Investigators
- 24.8% Success Rate (R01)
- 27.1% Early Stage Investigator Success Rate (R01)
- 180 Competing Research Project Grants
- 61 Competing Individual and Institutional Training Awards
Responding to COVID-19

- Lead on NIH's Rapid Acceleration of Diagnostics Radical initiatives to support development of quick and inexpensive tests to assess chemosensory function in symptomatic and asymptomatic individuals for COVID-19.
- Ensure research support to scientists and early career scientists whose career trajectories are significantly impacted by the pandemic. For example, NIDCD allowed generous no-cost extensions and modifications to existing grant protocols to allow virtual and telehealth approaches.
Brain Computer Interface
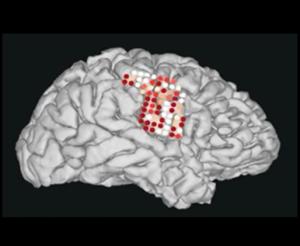
NIDCD invests in assistive communication devices, such as Brain Computer Interface (BCI) technology, to help people with severe speech and physical impairment regain their ability to communicate. BCI technology uses sensors to measure electrical signals in the brain to run a computer. Recently, NIDCD-supported engineers have successfully developed a "speech neuroprosthesis" that decodes a person's thoughts and turns them directly into speech.
Diversity, Equity, and Inclusion

NIDCD recognizes for the United States to remain a global leader in scientific discovery and innovation, it is dependent upon a pool of highly talented scientists from diverse backgrounds. NIDCD supports two Funding Opportunity Announcements where the overarching goal is to support educational and mentoring activities that encourage individuals from diverse backgrounds, including those from groups underrepresented in the biomedical and behavioral sciences, to pursue further studies or careers in research.
2022-2027 NIDCD Strategic Plan
With the guidance of our advisory committees and input from our public stakeholders, NIDCD will examine our current research portfolio and priorities, opportunities for growth and partnerships, and the impact on public health in developing the 2022-2027 NIDCD Strategic Plan with a vision toward the future. We aim to support innovative, cross-cutting, multi-disciplinary research so that all Americans can benefit from scientific discovery that will inform effective and accessible prevention strategies and treatments for deafness and other communication disorders.
Major Changes in the Fiscal Year 2023 President’s Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2023 President’s Budget for NIDCD, which is $10.6 million above the FY 2022 Continuing Resolution (CR) level, for a total of $508.7 million.
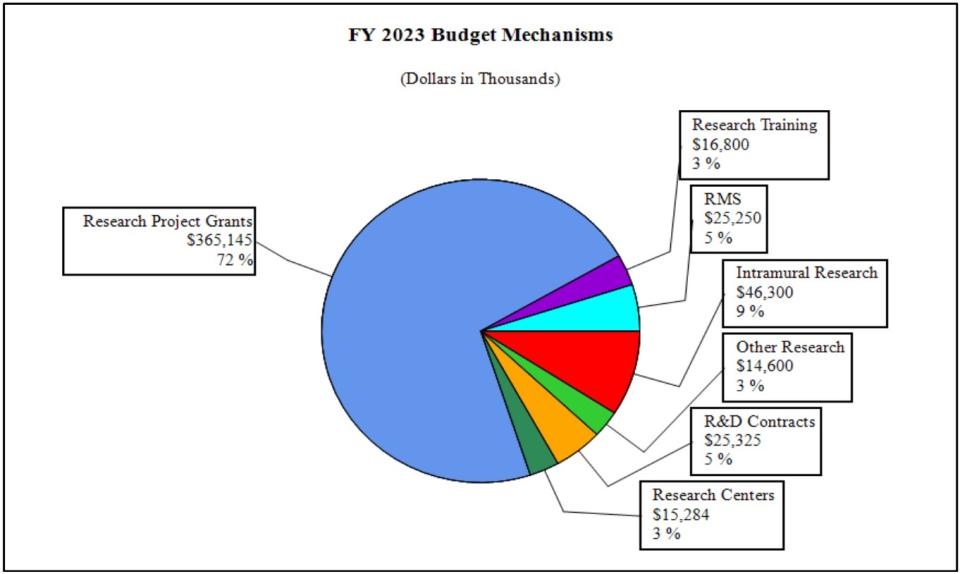
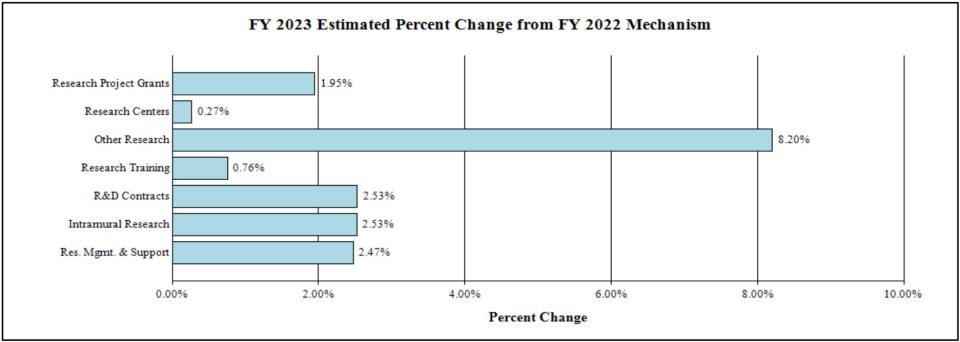
Research Project Grants (RPGs) (+$7.0 million; total $365.1 million):
NIDCD will fund 780 RPG awards in FY 2023, an increase of 1 from the FY 2022 CR level. This includes 590 non-competing awards (equal to the FY 2022 CR level) and 168 competing RPGs (equal to the FY 2022 CR level).
Other Research (+$1.1 million; total $14.6 million):
NIDCD will fund 94 Other Research awards in FY 2023, an increase of 4 from the FY 2022 CR level.
Intramural Research (+$1.1 million; total $46.3 million):
In addition to growth to cover pay increases and other inflationary costs in Intramural Research, in FY 2023 NIDCD will establish a new Neurotology Branch and lab.
| (Dollars in Thousands) | ||||||||
|---|---|---|---|---|---|---|---|---|
| MECHANISM | FY 2021 Final | FY 2022 CR | FY 2023 President's Budget | FY 2023 +/- FY 2022 CR | ||||
| No. | Amount | No. | Amount | No. | Amount | No. | Amount | |
| Research Projects: | ||||||||
| Noncompeting | 582 | $256,482 | 590 | $266,391 | 590 | $272,920 | 0 | $6,529 |
| Administrative Supplements | (60) | 6,524 | (40) | 2,000 | (40) | 2,000 | (0) | 0 |
| Competing: | ||||||||
| Renewal | 37 | 19,776 | 33 | 17,855 | 33 | 17,900 | 0 | 45 |
| New | 143 | 60,829 | 135 | 56,420 | 135 | 56,500 | 0 | 80 |
| Supplements | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subtotal, Competing | 180 | $80,605 | 168 | $74,275 | 168 | $74,400 | 0 | $125 |
| Subtotal, RPGs | 762 | $343,612 | 758 | $342,666 | 758 | $349,320 | 0 | $6,654 |
| SBIR/STTR | 21 | 15,492 | 21 | 15,500 | 22 | 15,825 | 1 | 325 |
| Research Project Grants | 783 | $359,103 | 779 | $358,166 | 780 | $365,145 | 1 | $6,979 |
| Research Centers: | ||||||||
| Specialized/Comprehensive | 7 | $17,942 | 6 | $15,212 | 6 | $15,253 | 0 | $41 |
| Clinical Research | 0 | 278 | 0 | 31 | 0 | 31 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative Medicine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers | 7 | $18,220 | 6 | $15,243 | 6 | $15,284 | 0 | $41 |
| Other Research: | ||||||||
| Research Careers | 57 | $9,375 | 56 | $9,333 | 56 | $9,400 | 0 | $67 |
| Cancer Education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 28 | 2,674 | 34 | 4,160 | 38 | 5,200 | 4 | 1,040 |
| Other Research | 85 | $12,048 | 90 | $13,493 | 94 | $14,600 | 4 | $1,107 |
| Total Research Grants | 875 | $389,371 | 875 | $386,902 | 880 | $395,029 | 5 | $8,127 |
| Ruth L Kirschstein Training Awards: | FTTPs | FTTPs | FTTPs | FTTPs | ||||
| Individual Awards | 156 | $7,359 | 155 | $7,357 | 156 | $7,450 | 1 | $93 |
| Institutional Awards | 157 | 8,892 | 162 | 9,317 | 162 | 9,350 | 0 | 33 |
| Total Research Training | 313 | $16,252 | 317 | $16,674 | 318 | $16,800 | 1 | $126 |
| Research & Develop. Contracts | 43 | $21,907 | 44 | $24,700 | 44 | $25,325 | 0 | $625 |
| (SBIR/STTR) (non-add) | (0) | (161) | (0) | (161) | (0) | (161) | (0) | (0) |
| Intramural Research | 60 | 44,747 | 64 | 45,159 | 64 | 46,300 | 0 | 1,141 |
| Res. Management & Support | 74 | 24,301 | 76 | 24,641 | 76 | 25,250 | 0 | 609 |
| SBIR Admin. (non-add) | (0) | (0) | (0) | (0) | (0) | (0) | (0) | (0) |
| Construction | 0 | 0 | 0 | 0 | ||||
| Buildings and Facilities | 0 | 0 | 0 | 0 | ||||
| Total, NIDCD | 134 | $496,578 | 140 | $498,076 | 140 | $508,704 | 0 | $10,628 |
| 1All items in italics and brackets are non-add entries | ||||||||
NATIONAL INSTITUTE ON DEAFNESS AND OTHER COMMUNICATION DISORDERS
For carrying out section 301 and title IV of the PHS Act with respect to deafness and other communication disorders, $508,704,000.
| (Dollars in Thousands) | ||||||
|---|---|---|---|---|---|---|
| FY 2022 CR | $498,076 | |||||
| FY 2023 President's Budget | $508,704 | |||||
| Net change | $10,628 | |||||
| CHANGES | FY 2022 CR | FY 2023 President's Budget | Built-In Change from FY 2022 CR | |||
| FTEs | Budget Authority | FTEs | Budget Authority | FTEs | Budget Authority | |
| A. Built-in: | ||||||
| 1. Intramural Research: | ||||||
| a. Annualization of January 2022 pay increase & benefits | $12,759 | $13,227 | $85 | |||
| b. January FY 2023 pay increase & benefits | 12,759 | 13,227 | 432 | |||
| c. Paid days adjustment | 12,759 | 13,227 | -48 | |||
| d. Differences attributable to change in FTE | 12,759 | 13,227 | 0 | |||
| e. Payment for centrally furnished services | 7,571 | 7,722 | 151 | |||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | 24,829 | 25,351 | 521 | |||
| Subtotal | $1,141 | |||||
| 2. Research Management and Support: | ||||||
| a. Annualization of January 2022 pay increase & benefits | $15,141 | $15,695 | $100 | |||
| b. January FY 2023 pay increase & benefits | 15,141 | 15,695 | 512 | |||
| c. Paid days adjustment | 15,141 | 15,695 | -58 | |||
| d. Differences attributable to change in FTE | 15,141 | 15,695 | 0 | |||
| e. Payment for centrally furnished services | 2,221 | 2,265 | 44 | |||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | 7,279 | 7,290 | 154 | |||
| Subtotal | $753 | |||||
| Subtotal, Built-in | $1,894 | |||||
| CHANGES | FY 2022 CR | FY 2023 President's Budget | Program Change from FY 2022 CR | |||
|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | |
| B. Program: | ||||||
| 1. Research Project Grants: | ||||||
| a. Noncompeting | 590 | $268,391 | 590 | $274,920 | 0 | $6,529 |
| b. Competing | 168 | 74,275 | 168 | 74,400 | 0 | 125 |
| c. SBIR/STTR | 21 | 15,500 | 22 | 15,825 | 1 | 325 |
| Subtotal, RPGs | 779 | $358,166 | 780 | $365,145 | 1 | $6,979 |
| 2. Research Centers | 6 | $15,243 | 6 | $15,284 | 0 | $41 |
| 3. Other Research | 90 | 13,493 | 94 | 14,600 | 4 | 1,107 |
| 4. Research Training | 317 | 16,674 | 318 | 16,800 | 1 | 126 |
| 5. Research and development contracts | 44 | 24,700 | 44 | 25,325 | 0 | 625 |
| Subtotal, Extramural | $428,276 | $437,154 | $8,878 | |||
| FTEs | FTEs | FTEs | ||||
| 6. Intramural Research | 64 | $45,159 | 64 | $46,300 | 0 | $0 |
| 7. Research Management and Support | 76 | 24,641 | 76 | 25,250 | 0 | -144 |
| 8. Construction | 0 | 0 | 0 | |||
| 9. Buildings and Facilities | 0 | 0 | 0 | |||
| Subtotal, Program | 140 | $498,076 | 140 | $508,704 | 0 | $8,734 |
| Total built-in and program changes | $10,628 | |||||
Fiscal Year 2023 Budget Graphs
History of Budget Authority and FTEs:
Distribution by Mechanism:
Change by Selected Mechanisms:
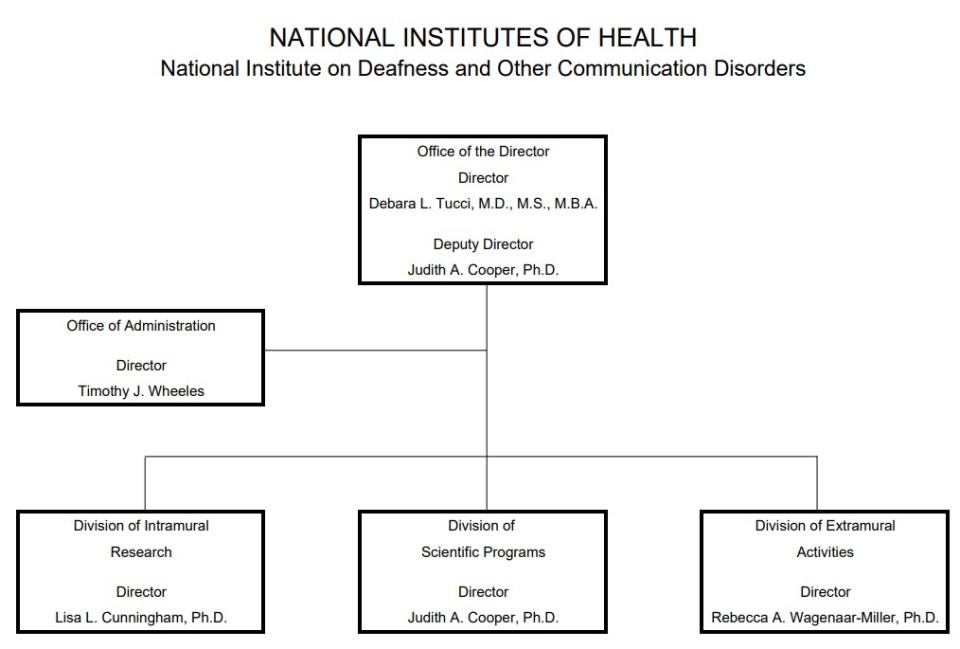
Organization Chart
| (Dollars in Thousands) | ||||||||
|---|---|---|---|---|---|---|---|---|
| FY 2021 Final | FY 2022 CR | FY 2023 President's Budget | FY 2023 +/- FY 2022 CR | |||||
| Extramural Research | FTE | Amount | FTE | Amount | FTE | Amount | FTE | Amount |
| Detail | ||||||||
| Hearing and Balance | $229,347 | $229,747 | $234,509 | $4,763 | ||||
| Taste and Smell | 62,494 | 62,603 | 63,901 | 1,298 | ||||
| Voice, Speech, and Language | 135,690 | 135,926 | 138,744 | 2,818 | ||||
| Subtotal, Extramural | $427,530 | $428,276 | $437,154 | $8,878 | ||||
| Intramural Research | 60 | $44,747 | 64 | $45,159 | 64 | $46,300 | 0 | $1,141 |
| Research Management & Support | 74 | $24,301 | 76 | $24,641 | 76 | $25,250 | 0 | $609 |
| TOTAL | 134 | $496,578 | 140 | $498,076 | 140 | $508,704 | 0 | $10,628 |
| 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | ||||||||
| FY 2021 Final | FY 2022 Continuing Resolution | FY 2023 President's Budget | FY 2023 +/- FY 2022 | |
|---|---|---|---|---|
| BA | $496,578,000 | $498,076,000 | $508,704,000 | +$10,628,000 |
| FTE | 134 | 140 | 140 | 0 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Overall Budget Policy: The FY 2023 President’s Budget request for NIDCD is $508.7 million, an increase of $10.6 million or 2.1 percent compared with the FY 2022 CR level.
Program Descriptions
Hearing and Balance Program: The human middle and inner ear are unusually hard to study as the ear is encased in the dense temporal bone of the skull; moreover, the soft tissues cannot be readily accessed by routine biopsy or other techniques on living subjects. Studies of human auditory and vestibular tissues are limited by the uneven quality of preservation and preparation of donated post-mortem specimens. These restrictions seriously limit progress in understanding pathology of the organs of hearing and balance in humans. To facilitate human temporal bone research, NIDCD supports two resources for scientific researchers through cooperative agreement awards. The NIDCD National Temporal Bone, Hearing, Balance Pathology Resource Registry, housed at Mass General Brigham, Mass Eye and Ear, serves to recruit new temporal bone donors, coordinates the procurement of the donated specimens upon death of the donor, and maintains an extensive searchable database of existing temporal bone specimens.13 Today, it is the home to more than 12,000 temporal bone cases, which scientists from around the globe can analyze in order to find new causes, treatments, and cures for problems associated with hearing, balance, and the facial nerve. The NIDCD Temporal Bone Laboratory at the University of California Los Angeles is devoted to the microscopic study of the ears of individuals who have suffered from a hearing or balance problem and have willed their temporal bones, containing the ear structures, for scientific analysis.14 By comparing abnormal microscopic findings in a large number of temporal bones from individuals with similar clinical findings, scientists can learn the underlying anatomical abnormality responsible for a particular disability. The laboratory is a resource for researchers to obtain human temporal bone specimens and assistance with techniques for handling and staining the specimens. Through these resources, researchers have access to human inner and middle ear tissues to investigate differences between normal and disordered tissue or to validate if studies of animals with hearing and balance disorders can provide information on how these disorders develop in humans. It is important to confirm that the hypotheses generated from animal models hold true in humans before beginning to consider gene therapy or other treatments. Access to human auditory and vestibular tissue with known medical histories is an important tool to be able to make that translational step.
EXPERT PANEL ON OTITIS MEDIA RESEARCH
Otitis media (OM), commonly known as ear infections, represents a substantial disease burden in the United States and globally, especially in underserved populations. In the United States, OM is the most common childhood illness, with more than 90 percent of children having an episode by age 5, and roughly 15 percent experiencing one of the more serious forms, such as recurrent or chronic OM. The impact of recurring or chronic OM on a child’s development can be significant, leading in some cases to hearing loss and to deficits in speech, language acquisition, learning, and complex auditory processing.
Given the public health burden of OM, NIDCD convened a group of experts for a virtual workshop in 2020 to collect scientific input on ways to advance OM research in early childhood, with an objective of identifying strategies to accelerate the transition from the basic discovery phase to disease prevention and therapies.
The experts identified major gaps in our understanding of OM that present opportunities for novel and continued research, as well as important barriers to progress in the field, in basic, clinical, and translation research. A report was submitted to NIDCD with the goal of significantly advancing the Institute’s portfolio of OM research. NIDCD staff will use the workshop panel’s recommendations and identify strategies that have the greatest potential to advance OM research and lead to more effective prevention and treatment approaches.
Goals of the workshop include helping raise awareness of the impact of this serious and common condition, attracting trainees and established scientists to the field, and inspiring innovative approaches to future OM research.
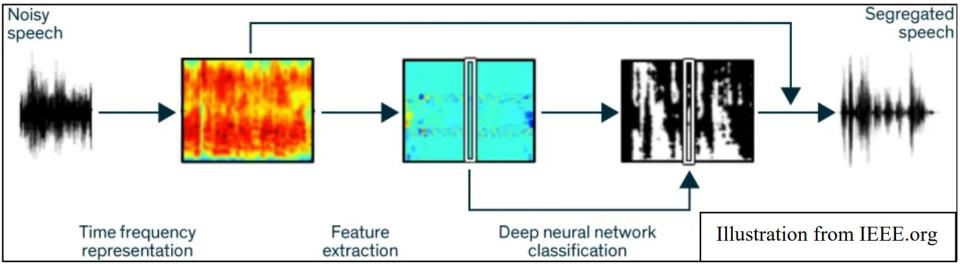
NIDCD supports research that aims to improve hearing aid technology such as applying new signal processing strategies to the design of hearing aids. Signal processing is the method used to modify normal sound waves into amplified sound that is the best possible match to the remaining hearing for a hearing aid user. A standard complaint from hearing aid users relates to the difficulty of understanding speech in the presence of background noise. This can occur when there are multiple talkers, such as in a restaurant. Recently, NIDCD-funded researchers used a deep computational auditory scene analysis (CASA) algorithm to improve sentence comprehension for hearing-impaired listeners in noisy environments.15 Researchers applied machine learning algorithms based on ‘deep neural networks’ to segregate noisy speech sounds in order to isolate and amplify a desired speech sound. The ‘deep neural network’ is trained to decipher speech (feature extraction) from other noises and can further ‘learn’ by adjusting soundwave input within the program. Once identified, the desired, segregated speech signal is selectively amplified. Further, when comparing intelligibility scores, hearing-impaired listeners with the CASA hearing aid technology could hear better than normal hearing listeners, without the technology, in the same listening environment.
Balance disorders affect a large proportion of the population, particularly the elderly. According to the Centers for Disease Control and Prevention (CDC), the estimated medical cost of falls across the U.S. healthcare system is $50 billion annually.16 NIDCD, in conjunction with the National Institute on Aging, is supporting a new initiative to understand the nervous system’s control of balance in older adults.17 In addition to the hearing organ (cochlea), the inner ear contains the vestibular system, which is responsible for our sense of balance. There are major gaps in our understanding of how aging impacts the vestibular system, which, when impaired, contributes to balance problems and an increased risk of falls in older adults, a significant public health issue. Given that balance impairment can be attributed to a number of factors (e.g., sensory, motor, cognitive, psychological, and vascular function), understanding the contribution of age-related vestibular dysfunction, and how this interacts with these factors, is critical for informing interventions and treatments for balance problems and falls in older adults. One NIDCD-funded study is currently investigating fall-related injuries in older adults with hearing loss.18 It aims to determine the relationship between falls, the level of hearing loss, and the type of amplification device. In doing so, researchers hope to determine an appropriate intervention to reduce falls in this group.
Budget Policy: The FY 2023 President’s Budget request for the hearing and balance program is $234.5 million, an increase of $4.8 million or 2.1 percent compared to the FY 2022 CR level.
Taste and Smell Program: Taste and smell disorders are underreported, and often undiagnosed. During the COVID-19 pandemic, the impact of taste and smell problems gained international attention because loss of taste and smell are frequent symptoms of COVID-19.19 NIDCD supports studies of the chemical senses known as taste, smell, and chemesthesis (chemically provoked irritation) to enhance our understanding of how individuals gather information about their environment and how human chemosensory disorders can be diagnosed and treated.
Insect bites spread diseases such as malaria, yellow fever, Zika, dengue, chikungunya, and Lyme disease, which take a heavy toll on human health. Insects track odors to target their prey, so understanding how insects detect odors is important to help stop the spread of insect-borne illnesses. NIDCD-supported scientists are using cryo-electron microscopy to understand how odorant molecules bind to insect odorant receptors and alert the insect to an odor.20 The research team compared the shape of an insect odorant receptor before and after binding the odorant, eugenol, and then before and after binding the insect repellent DEET. Rather than tightly and specifically binding one particular odorant, the insect odorant receptor loosely binds a wide array of odorants. This new research helps explain how insects with a limited number of olfactory receptors can detect a very broad array of odorants. Future research may help us understand how to use this new knowledge to develop better insect repellants, thus helping curb the spread of insect-borne disease.
Humans and animals learn to avoid poisonous food by associating that food with a negative consequence, such as stomach discomfort in a process called conditioned taste aversion (CTA). CTA learning involves the brain making connections between the taste region, called the gustatory cortex, and the amygdala, the part of the brain that is involved in positive and negative “emotion.” Recently, NIDCD-supported researchers studied CTA learning in rats to better understand how CTA rewires brain connections.21 Rats find drinking sugar water very rewarding and prefer it to plain water. In this experiment, scientists allowed rats to drink sugar water, but paired it with the delivery of a chemical called lithium chloride, which caused temporary nausea. The next day, the rats that had received lithium chloride avoided sugar water in favor of plain water. In other words, they learned to avoid something they usually find very rewarding. To investigate how this behavior learning changed the brain, the researchers measured electrical activity in the gustatory cortex and in the amygdala. They observed reduced electrical connections between the taste cortex and the amygdala in rats that learned to avoid sugar water through CTA. More amazingly, the team was able to reproduce this phenomenon in the rat brain in the absence of the behavioral training by using optogenetic (light energy to turn brain cells on or off) stimulation to reduce the strength of connections between the amygdala and the gustatory cortex. Understanding how we can alter positive and negative associations to foods and drugs have broader implications for helping people break addiction to harmful substances.

Budget Policy: The FY 2023 President’s Budget request for the taste and smell program is $63.9 million, an increase of $1.3 million or 2.1 percent compared to the FY 2022 CR level.
NEW TASTE AND SMELL TESTS TO SCREEN FOR COVID-19
Sudden loss of smell or taste is recognized as one of the hallmarks of COVID-19 by the CDC. Up to 80 percent of patients infected with SARS-CoV-2 report a new partial or complete loss of smell or taste. In fact, recent observational studies indicate that chemosensory loss is one of the most common symptoms of COVID-19 and more predictive of infection than all other symptoms, including fatigue, fever, or cough.
As part of the NIH Rapid Acceleration of Diagnostics Radical (RADx-rad) Program, NIDCD is funding four new projects developing smell and taste tests to screen for COVID-19. These projects focus on developing new tests that are cost-effective, stable, rapid, and readily scalable, allowing them to be used in any setting, including at home, at large venues, in schools, and in under-resourced communities. Potentially, the tests can help us globally track disease outbreaks and address healthcare needs for people impacted by this pandemic.
The projects are:
- A confectionary-based screening tool for assessing chemosensory loss in COVID-19 patients. Uses hard candy to assess smell and taste function in individuals who are at high risk for contracting COVID-19.
- SCENTinel: A rapid smell test for COVID-19 surveillance. Deploys the SCENTinel test, a rapid and objective measure of smell ability for widespread COVID-19 surveillance.
- Rapid olfactory tools for telemedicine-friendly COVID-19 screening and surveillance. Self-administered smell tests to identify people with COVID-19 prior to, or in the absence of, viral testing, as well as for use in population-level surveillance of COVID-19 spread.
- Longitudinal at-home smell testing to detect infection by SARS-CoV-2. App to conduct longitudinal smell tests and differentiate smell loss due to COVID-19 versus influenza and assess the risk of pulmonary disease in people with COVID-19.
Voice, Speech, and Language Program: Disorders involving voice, speech, or language can have an overwhelming effect on an individual’s health and quality of life. These disorders affect people of all ages with or without hearing impairment, such as children with autism, people who stutter, and adults with acquired communication disorders. Voice, speech, and language disorders also come at a significant cost. The cost to society of laryngeal disorders is estimated at $11 billion annually due to work-related disability, lost productivity, and direct health care cost. Further, nearly eight percent of children ages 3-17 years have had a communication disorder during the past 12 months, according to data from the National Health Interview Survey, 2012. By the first grade, roughly five percent of children have noticeable speech disorders. In children, delayed speech and language acquisition or impairment are often significant predictors of future academic, social, vocational, and adaptive outcomes.
Laryngeal dystonia (LD), also referred to as spasmodic dysphonia, is a voice disorder that belongs to a family of neurological disorders called focal dystonias. When a person with LD attempts to speak, the muscles in the larynx spasm involuntarily and cause the voice to break up and sound strained or breathy. Currently, there is no cure for LD, and the most common treatments only offer temporary relief of symptoms. In 2019, the NIDCD convened a multidisciplinary panel of experts to examine the current progress in understanding the causes of LD and to delineate research priorities for improving clinical care.22 Why an individual may develop LD remains unknow, and the experts agreed the pathology of the disorder likely involves large-scale functional and structural brain network disorganization. Clinical challenges include the lack of validated diagnostic markers and outcome measures, and the paucity of therapies that address the disordered pathology. NIDCD currently funds research to better understand LD, determine its causes, and develop new diagnostics and better treatment options. NIDCD-supported scientists are conducting research to identify brain abnormalities and genes that cause LD. NIDCD-funded scientists are also pursuing several new areas for future therapies and surgical interventions, including locating specific brain areas involved in regulating abnormal laryngeal muscle activity and looking for new oral medications that affect the abnormal brain activity underlying LD. Through this research program, NIDCD is directly addressing the need for more accurate detection, prediction, and diagnosis.

Dysarthria is a communication disorder caused by impairments to the parts of the brain that control the muscles in the lips, tongue, vocal folds, and diaphragm. The muscles are weakened and result in slurred or slow speech, which makes it difficult for an individual to be understood. NIDCD supports research to develop tools for better diagnosis and interventions for individuals with dysarthria. For example, one research study is investigating speech and language development in children with cerebral palsy (CP).23 Around 60 percent of children with CP have some type of communication disorder, including dysarthria; however, very little is known about the specific nature of these problems, how they change as children grow, or how to treat them. The scientists will collect over 15 years of data with the goal of developing the predictive ability to determine speech and language outcomes for children. These outcome trajectories are necessary to develop interventions that improve speech, language, and communication, and ultimately quality of life for children with CP.
Autism spectrum disorders (ASD) are a group of developmental disabilities that can cause significant social, communication, and behavioral challenges. According to the Centers for Disease Control and Prevention (CDC), about 1 in 54 children is diagnosed with ASD and 25-30 percent of children with ASD remain functionally non-verbal beyond 5 years of age – a subset under-represented in autism research. NIDCD supports research that looks at speech and language abilities and differences in individuals with ASD. NIDCD also supports research efforts to improve the early diagnosis of ASD and to develop effective treatments to address the communication challenges faced by many with ASD. For example, a recently funded clinical research project focuses on minimally verbal children with autism.24 While the majority of children with ASD who have not acquired spoken language by age four remain minimally verbal over the long term, a minority are ‘late bloomers’ and make significant gains despite their earlier delays. The research team aims to explore who these children are, and how we might optimize their language development by investigating genetics, motor and brain functioning, and evaluating a novel behavioral treatment. The findings will have an important impact on clinical practice and will lead to new ways of identifying the children with ASD most at risk for severe communication disorders.
Budget Policy: The FY 2023 President’s Budget request for the voice, speech, and language program is $138.7 million, an increase of $2.8 million or 2.1 percent compared with the FY 2022 CR level.
Intramural Research Program: The NIDCD Intramural Research Program conducts basic and clinical research in human communication, with a primary focus on hearing and balance.
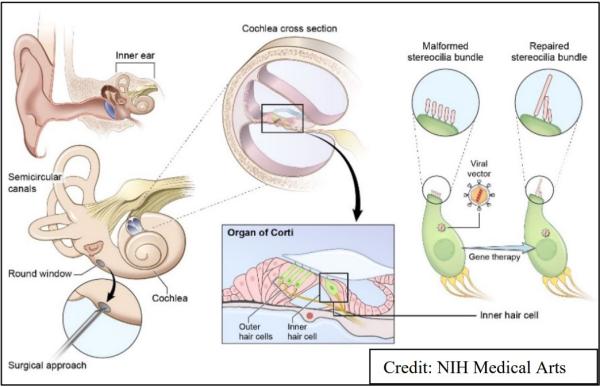
One intramural laboratory is developing gene therapies aimed at correcting hereditary hearing loss. Most current gene therapy research relies on viruses to deliver new genes to cells because viruses naturally infect cells by injecting their own genetic material into them. A type of virus called an adeno-associated virus (AAV) is one of the most commonly used viruses in gene therapy research. However, naturally occurring AAVs have significant limitations when it comes to gene therapy for hearing loss: many of them are good at infecting only one of the two types of sensory cells that are critical for hearing, called inner hair cells, but not the other, called outer hair cells. In a new study, NIDCD researchers tested the ability of several AAVs – some naturally occurring and some artificially created – to deliver genes to several types of cells in the inner ears of mice.25 For the purposes of this experiment, the viruses inserted a gene that produced a fluorescent protein, making it easy for the researchers to see which cells were infected by the virus. One artificially-designed virus called AAV2.7m8 delivered the gene to over 80 percent of both cell types.26 It also infected the hair cells in the inner ear that are responsible for balance. AAV2.7m8 did not appear to damage the cells it infected, suggesting that it may be safe for use in human gene therapy studies. Researchers must next test if AAV2.7m8 can deliver corrected hearing genes in animal models of hearing loss and whether these genes can restore hearing before they can begin to develop human clinical trials.

Many military personnel are injured by blast waves from explosions during combat that lead to problems with hearing and balance, but little is known about how blast waves cause those problems. NIDCD intramural scientists, along with researchers at Walter Reed National Military Medical Center, performed experiments in animal models to understand how this happens. In a new study, they exposed mice to simulated blasts and then assessed the effects on hearing and balance over six months.27 They found that blast exposure can cause severe, long-term hearing loss, which may be due, in part, to slow healing of the eardrums or altered function of healed eardrums. They also found that not only does blast exposure rupture the ear drum, but it can also directly impact the sensory hair cells of the inner ear. This suggests that hearing protection (like earplugs) may not be enough to protect against sensory hair cell loss in some situations. A third finding was that there was significant loss of connections between hair cells and the spiral ganglion neurons that transmit sound information to the brain. This replicates a phenomenon thought to occur in humans, where hair cells are alive, but the connection to the neurons may be impaired and thus hearing is affected. Finally, the researchers found that balance deficits after blast exposure may be primarily due to traumatic brain injury, rather than damage to the part of the inner ear that is necessary for balance. Understanding how blast waves cause problems to hearing and balance will help direct future studies on how best to prevent and treat problems for military personnel who return from combat.
Another NIDCD intramural laboratory is working to prevent hearing loss caused by cisplatin—a life-saving cancer drug. While cisplatin is rapidly cleared from the body, it builds up in the inner ear and kills the sensory hair cells that detect sound. Once lost, auditory hair cells in mammals are not spontaneously repaired or replaced, resulting in hearing loss. Previous research from this team showed that FDA-approved statin medications, taken by more than 35 million Americans to lower cholesterol, also preserve hearing in mice treated with cisplatin therapy. Now the researchers have begun to extend these findings to humans by examining hearing function in people treated with cisplatin who are also taking a statin drug.28 The researchers found that cisplatin therapy resulted in hearing loss in about half of individuals with head and neck cancer, and they observed reduced occurrence and severity of cisplatin-induced hearing loss in people taking a statin called atorvastatin relative to those not taking a statin drug. Statins are inexpensive, well-tolerated, and do not reduce the therapeutic efficacy of cisplatin. The team has now started a Phase III clinical trial to determine if atorvastatin can help prevent cisplatin-induced hearing loss in people being treated for cancer.29 Scientists hope these findings can quickly translate into prevention strategies for young children with cancer receiving cisplatin.
Budget Policy: The FY 2023 President’s Budget request for the intramural research program is $46.3 million, an increase of $1.1 million or 2.5 percent compared with the FY 2022 CR level.
Research Management and Support (RMS) Program: NIDCD RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS functions also include strategic planning, coordination, and evaluation of the Institute’s programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. The Institute currently supports approximately 1,400 research grants, training awards, and Research & Development contracts.
NIDCD is working to increase the diversity of the research pipeline across its mission areas. In 2021, NIDCD established the NIDCD Diversity Working Group to reinvigorate the Institute’s commitment to build and sustain a diverse research workforce. It will work with NIDCD leadership to propose how the basic tenets of diversity, inclusion, equity, and respect could be better realized within NIDCD. The Working Group will specifically address how to increase and showcase diversity at all levels within the Institute and explore and implement opportunities to improve inclusion, equity, and respect, and foster an informed community that is welcoming to everyone. The NIDCD Diversity Working Group is the only internal trans-NIDCD committee and is charged to integrate suggestions on diversity, equity, inclusion, and accessibility from within the Institute, including the National Deafness and Other Communication Disorders Advisory Council, as well as provide specific policy recommendations for the Institute that no one NIDCD Division or trans-NIH group can.
Budget Policy: The FY 2023 President’s Budget request for RMS is $25.3 million, an increase of $0.6 million or 2.5 percent compared with the FY 2022 CR level.
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation | ||||
|---|---|---|---|---|---|---|---|---|
| 2014 | $422,936,000 | $420,125,000 | $404,049,000 | |||||
| Rescission | $0 | |||||||
| 2015 | $403,933,000 | $405,302,000 | ||||||
| Rescission | $0 | |||||||
| 2016 | $416,241,000 | $412,366,000 | $424,860,000 | $423,031,000 | ||||
| Rescission | $0 | |||||||
| 2017[1] | $422,936,000 | $434,126,000 | $441,778,000 | $436,875,000 | ||||
| Rescission | $0 | |||||||
| 2018 | $325,846,000 | $443,624,000 | $451,768,000 | $459,974,000 | ||||
| Rescission | $0 | |||||||
| 2019 | $423,992,000 | $465,467,000 | $474,653,000 | $474,404,000 | ||||
| Rescission | $0 | |||||||
| 2020 | $408,358,000 | $497,590,000 | $500,270,000 | $490,692,000 | ||||
| Rescission | $0 | |||||||
| 2021 | $446,397,000 | $494,912,000 | $506,670,000 | $498,076,000 | ||||
| Rescission | $0 | |||||||
| 2022 | $511,792,000 | $522,758,000 | $511,280,000 | $498,076,000 | ||||
| Rescission | $0 | |||||||
| 2023 | $508,704,000 | |||||||
| 1 Budget Estimate to Congress includes mandatory financing. | ||||||||
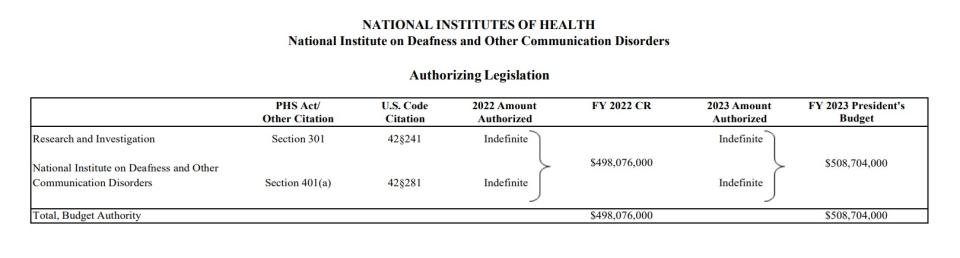
Authorizing Legislation
| (Dollars in Thousands) | |||
|---|---|---|---|
| Source of Funding | FY 2021 Final | FY 2022 CR | FY 2023 President's Budget |
| Appropriation | $498,076 | $498,076 | $508,704 |
| Mandatory Appropriation: (non-add) | |||
| Secretary's Transfer | -1,495 | 0 | 0 |
| OAR HIV/AIDS Transfers | -3 | 0 | 0 |
| Subtotal, adjusted budget authority | $496,578 | $498,076 | $508,704 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year (carryover) | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | $496,578 | $498,076 | $508,704 |
| Unobligated balance lapsing | -4 | 0 | 0 |
| Total obligations | $496,574 | $498,076 | $508,704 |
| 1 Excludes the following amounts (in thousands) for reimbursable activities carried out by this account: FY 2021 - $484 FY 2022 - $1,000 FY 2023 - $1,000 | |||
| (Dollars in Thousands) | ||||
|---|---|---|---|---|
| FY 2022 CR | FY 2023 President's Budget | FY 2023 +/- FY 2022 CR | ||
| Total compensable workyears: | ||||
| Full-time equivalent | 140 | 140 | 0 | |
| Full-time equivalent of overtime and holiday hours | 0 | 0 | 0 | |
| Average ES salary | $204 | $213 | $9 | |
| Average GM/GS grade | 12.6 | 12.6 | 0.0 | |
| Average GM/GS salary | $128 | $134 | $6 | |
| Average salary, Commissioned Corps (42 U.S.C. 207) | $142 | $148 | $7 | |
| Average salary of ungraded positions | $126 | $132 | $6 | |
| OBJECT CLASSES | FY 2022 CR | FY 2023 President's Budget | FY 2023 +/- FY 2022 | |
| Personnel Compensation | ||||
| 11.1 | Full-Time Permanent | 13,735 | 14,249 | 514 |
| 11.3 | Other Than Full-Time Permanent | 5,149 | 5,342 | 193 |
| 11.5 | Other Personnel Compensation | 600 | 622 | 22 |
| 11.7 | Military Personnel | 141 | 146 | 5 |
| 11.8 | Special Personnel Services Payments | 1,228 | 1,274 | 46 |
| 11.9 | Subtotal Personnel Compensation | $20,853 | $21,633 | $780 |
| 12.1 | Civilian Personnel Benefits | 6,984 | 7,223 | 239 |
| 12.2 | Military Personnel Benefits | 64 | 66 | 2 |
| 13.0 | Benefits to Former Personnel | 0 | 0 | 0 |
| Subtotal Pay Costs | $27,900 | $28,922 | $1,022 | |
| 21.0 | Travel & Transportation of Persons | 9 | 9 | 0 |
| 22.0 | Transportation of Things | 37 | 37 | 1 |
| 23.1 | Rental Payments to GSA | 0 | 0 | 0 |
| 23.2 | Rental Payments to Others | 0 | 0 | 0 |
| 23.3 | Communications, Utilities & Misc. Charges | 24 | 25 | 1 |
| 24.0 | Printing & Reproduction | 2 | 2 | 0 |
| 25.1 | Consulting Services | 10,499 | 10,710 | 211 |
| 25.2 | Other Services | 7,937 | 8,113 | 175 |
| 25.3 | Purchase of Goods and Services from Government Accounts | 38,249 | 39,148 | 898 |
| 25.4 | Operation & Maintenance of Facilities | 424 | 424 | 0 |
| 25.5 | R&D Contracts | 3,414 | 3,489 | 75 |
| 25.6 | Medical Care | 172 | 179 | 7 |
| 25.7 | Operation & Maintenance of Equipment | 1,608 | 1,500 | -108 |
| 25.8 | Subsistence & Support of Persons | 0 | 0 | 0 |
| 25.0 | Subtotal Other Contractual Services | $62,303 | $63,562 | $1,259 |
| 26.0 | Supplies & Materials | 2,234 | 2,283 | 49 |
| 31.0 | Equipment | 1,991 | 2,034 | 44 |
| 32.0 | Land and Structures | 0 | 0 | 0 |
| 33.0 | Investments & Loans | 0 | 0 | 0 |
| 41.0 | Grants, Subsidies & Contributions | 403,576 | 411,829 | 8,253 |
| 42.0 | Insurance Claims & Indemnities | 0 | 0 | 0 |
| 43.0 | Interest & Dividends | 0 | 0 | 0 |
| 44.0 | Refunds | 0 | 0 | 0 |
| Subtotal Non-Pay Costs | $470,176 | $479,782 | $9,606 | |
| Total Budget Authority by Object Class | $498,076 | $508,704 | $10,628 | |
| 1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | ||||
| (Dollars in Thousands) | |||
|---|---|---|---|
| OBJECT CLASSES | FY 2022 CR | FY 2023 President's Budget | FY 2023 +/- FY 2022 |
| Personnel Compensation | |||
| Full-Time Permanent (11.1) | $13,735 | $14,249 | $514 |
| Other Than Full-Time Permanent (11.3) | 5,149 | 5,342 | 193 |
| Other Personnel Compensation (11.5) | 600 | 622 | 22 |
| Military Personnel (11.7) | 141 | 146 | 5 |
| Special Personnel Services Payments (11.8) | 1,228 | 1,274 | 46 |
| Subtotal Personnel Compensation (11.9) | $20,853 | $21,633 | $780 |
| Civilian Personnel Benefits (12.1) | $6,984 | $7,223 | $239 |
| Military Personnel Benefits (12.2) | 64 | 66 | 2 |
| Benefits to Former Personnel (13.0) | 0 | 0 | 0 |
| Subtotal Pay Costs | $27,900 | $28,922 | $1,022 |
| Travel & Transportation of Persons (21.0) | $9 | $9 | $0 |
| Transportation of Things (22.0) | 37 | 37 | 1 |
| Rental Payments to Others (23.2) | 0 | 0 | 0 |
| Communications, Utilities & Misc. Charges (23.3) | 24 | 25 | 1 |
| Printing & Reproduction (24.0) | 2 | 2 | 0 |
| Other Contractual Services: | |||
| Consultant Services (25.1) | 10,499 | 10,710 | 211 |
| Other Services (25.2) | 7,937 | 8,113 | 175 |
| Purchases from Government Accounts (25.3) | 19,951 | 21,595 | 1,644 |
| Operation & Maintenance of Facilities (25.4) | 424 | 424 | 0 |
| Operation & Maintenance of Equipment (25.7) | 1,608 | 1,500 | -108 |
| Subsistence & Support of Persons (25.8) | 0 | 0 | 0 |
| Subtotal Other Contractual Services | $40,419 | $42,341 | $1,923 |
| Supplies & Materials (26.0) | $2,234 | $2,283 | $49 |
| Subtotal Non-Pay Costs | $42,724 | $44,698 | $1,973 |
| Total Administrative Costs | $70,625 | $73,620 | $2,995 |
| OFFICE/DIVISION | FY 2021 Final | FY 2022 CR | FY 2023 President's Budget | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Division of Extramural Activities | |||||||||
| Direct: | 18 | - | 18 | 18 | - | 18 | 18 | - | 18 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 18 | - | 18 | 18 | - | 18 | 18 | - | 18 |
| Division of Intramural Research Program | |||||||||
| Direct: | 59 | 1 | 60 | 63 | 1 | 64 | 63 | 1 | 64 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 59 | 1 | 60 | 63 | 1 | 64 | 63 | 1 | 64 |
| Division of Scientific Programs | |||||||||
| Direct: | 17 | - | 17 | 18 | - | 18 | 18 | - | 18 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 17 | - | 17 | 18 | - | 18 | 18 | - | 18 |
| Office of Administration | |||||||||
| Direct: | 37 | - | 37 | 38 | - | 38 | 38 | - | 38 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 37 | - | 37 | 38 | - | 38 | 38 | - | 38 |
| Office of the Director | |||||||||
| Direct: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Total: | 133 | 1 | 134 | 139 | 1 | 140 | 139 | 1 | 140 |
| Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||||||||
| FTEs supported by funds from Cooperative Research and Development Agreements. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FISCAL YEAR | Average GS Grade | ||||||||
| 2019 | 12.4 | ||||||||
| 2020 | 12.4 | ||||||||
| 2021 | 12.6 | ||||||||
| 2022 | 12.6 | ||||||||
| 2023 | 12.6 | ||||||||
| GRADE | FY 2021 Final | FY 2022 CR | FY 2023 President's Budget |
|---|---|---|---|
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | $199,300 | $203,700 | $213,070 |
| General Schedule | |||
| GM/GS-15 | 24 | 25 | 25 |
| GM/GS-14 | 20 | 20 | 20 |
| GM/GS-13 | 30 | 31 | 31 |
| GS-12 | 13 | 13 | 13 |
| GS-11 | 3 | 4 | 4 |
| GS-10 | 0 | 0 | 0 |
| GS-9 | 7 | 8 | 8 |
| GS-8 | 4 | 4 | 4 |
| GS-7 | 2 | 3 | 3 |
| GS-6 | 1 | 2 | 2 |
| GS-5 | 2 | 0 | 0 |
| GS-4 | 0 | 0 | 1 |
| GS-3 | 0 | 1 | 0 |
| GS-2 | 1 | 0 | 0 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 107 | 111 | 111 |
| Commissioned Corps (42 U.S.C. 207) | |||
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 0 | 0 | 0 |
| Senior Grade | 1 | 1 | 1 |
| Full Grade | 0 | 0 | 0 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 1 | 1 | 1 |
| Ungraded | 42 | 44 | 44 |
| Total permanent positions | 107 | 111 | 111 |
| Total positions, end of year | 151 | 157 | 157 |
| Total full-time equivalent (FTE) employment, end of year | 134 | 140 | 140 |
| Average ES salary | 199,300 | 203,700 | 213,070 |
| Average GM/GS Grade | 12.6 | 12.6 | 12.6 |
| Average GM/GS salary | 125,284 | 128,140 | 134,035 |
| [1] Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||
Footnotes
[1] NIDCD Epidemiology Branch

