- Download Congressional Justification 2022 (1.3MB PDF)
FY 2022 Budget
DEPARTMENT OF HEALTH AND HUMAN SERVICES
NATIONAL INSTITUTES OF HEALTH
National Institute on Deafness and Other Communication Disorders (NIDCD)
On this page:
- Director’s Overview
- IC Fact Sheet
- Major Changes in Budget Request
- Budget Mechanism Table
- Appropriations Language
- Summary of Changes
- Budget Graphs
- Organization Chart
- Budget Authority by Activity
- Justification of Budget Request
- Program Descriptions
- Appropriations History
- Authorizing Legislation
- Amounts Available for Obligation
- Budget Authority by Object Class
- Salaries and Expenses
- Detail of Full-Time Equivalent Employment (FTE)
- Detail of Positions
Director’s Overview

Dr. Debara Tucci, Director
At least 46 million people in the United States have a hearing or other communication disorder.[1] For these individuals, the basic components of communication—sensing, interpreting, and responding to people and things in our environment—can be challenging. The National Institute on Deafness and Other Communication Disorders (NIDCD) manages a broad portfolio of basic, translational, clinical, and public health research focused on human communication and associated disorders in three program areas: hearing and balance; taste and smell; and voice, speech, and language.
Capitalizing on Foundational Investments and Beyond: Extraordinary research opportunities have led to scientific breakthroughs in the study of genes, proteins, cellular and molecular processes, neural circuits, and sensory and motor systems that directly affect our understanding of deafness and other communication disorders. For example, NIDCD-supported research has led to advances in the following areas of communication science:
Developing Treatments for Communication Disorders Before Birth: In humans, the inner ear is developed by about the 17th week of pregnancy. Thus, there is a possibility to develop therapies that can target aberrant gene expression in the womb for congenital forms of hearing and balance disorders. To explore this, researchers funded in part by NIDCD injected a specially designed synthetic molecule, called antisense oligonucleotide (ASO), into the developing inner ear of fetal mice 12 days after fertilization.[2] At this timepoint, the hearing sensory cells are “born” but are not yet fully functional. ASOs by design are extremely precise and work by altering the way pre-messenger RNA is snipped and pasted together to make “mature” messenger RNA, which is then used as a template for the synthesis of a protein. Thus, correcting mutated forms of pre-messenger RNA restores healthy protein production. In this study, the scientists found that the technique corrected the protein expression of a mutated gene that causes Usher syndrome, a genetic disorder that can result in deafness and blindness. The mice were born and matured free of any symptoms of Usher syndrome. Researchers expect to move this technique from mice to nonhuman primates before it can be used in human clinical trials.
Basic Research Reveals How Coronavirus Enters the Respiratory System: Scientists have determined that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, which causes coronavirus disease 2019 (COVID-19), enters human cells by binding to an enzyme called ACE2, or angiotensin converting enzyme 2. Because humans inhale virus particles and the nasal lining is known to contain ACE2 receptors, NIDCD-supported scientists closely examined the lining of the nose to identify precisely which cell types express this enzyme and to what extent.[3],[4] One research team collected tissue from the nasal lining of individuals who were undergoing nasal surgery. They used molecules that bind to and label ACE2-containing cells and determined that ACE2 was mostly found in supportive cells known as sustentacular cells. Support cell infection may cause changes in odor-detecting cells that transmit information to the brain or can also result in the death of those neurons. The researchers propose that the abundant supply of ACE2 in the nose explains how inhaled SARS-CoV-2 virus particles gain a foothold in the early stages of COVID-19. They further suggest testing whether COVID-19 may be treated by adding anti-viral solutions to a nasal saline irrigation.
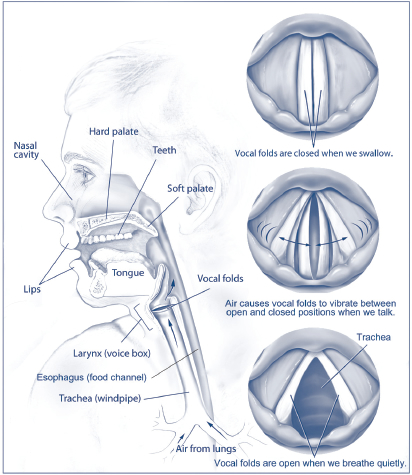
Using Adult Human Stem Cells to Develop Treatments for Voice Disorders: The sound of your voice is produced by vibration of the vocal folds. Vocal fold tissue is a complex biological structure, and people who have sustained injuries to the larynx or have undergone head and neck cancer surgery can exhibit voice disorders due to vocal fold dysfunction. These disorders can range in severity from mild to total voice loss. It is difficult to study treatment strategies for disorders of the larynx because taking tissue samples can cause irreversible damage, and reliable human tissue models do not exist. However, in a recent advance, NIDCD-funded researchers have created an in vitro model of vocal fold tissue from induced human pluripotent stem cells.[5] Further, when exposed to environmental insults like cigarette smoke, the model tissue had an inflammatory response like normal vocal fold tissue—an important feature for a model system. Currently, there are no treatments to restore vocal function for individuals who have voice disorders. These research findings are a critical first step for scientist to one day develop regenerative medicine treatments for injured or damaged vocal fold tissue.
Answering the Call: NIDCD continues its support of new and competing Research Project Grants from investigators who have innovative ideas. NIDCD prioritizes its research investments to identify the most promising opportunities for research on deafness and other communication disorders, including:
Research on Coronavirus Disease 2019 (COVID-19): NIDCD is actively pursuing new research applications to fill a knowledge gap on how COVID-19 impacts sensory and communication functions in hearing, balance, taste, smell, voice, speech, and language.[6],[7] NIDCD is particularly interested in understanding how COVID-19 causes loss of smell—further building on the basic research findings described above—and whether that loss of smell can be used to predict SARS-CoV-2 infection. We added supplemental funds to 17 NIDCD grants to enable them to address the pathology, prevention, diagnosis, sequelae, or treatment of COVID-19.[8] Further, NIDCD is the lead on one of the National Institutes of Health’s (NIH) Rapid Acceleration of Diagnostics Radical (RADx-rad) initiatives to support development of quick and inexpensive tests for COVID-19. Additional awards are supporting community and digital healthcare interventions to address the social, behavioral, and economic health impacts of COVID-19 in vulnerable and health disparate populations.[9]
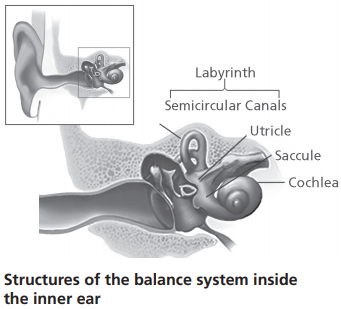
Control of Balance in Older Adults: NIDCD in conjunction with the National Institute on Aging (NIA) is supporting a new initiative to understand the nervous system’s control of balance in older adults.[10] In addition to the hearing organ (cochlea), the inner ear contains the vestibular system, which is responsible for our sense of balance. There are major gaps in our understanding of how aging impacts the vestibular system, which, when impaired, contributes to balance problems and an increased risk of falls in older adults, a significant public health issue. Given that balance impairment can be attributed to a number of factors (e.g., sensory, motor, cognitive, psychological, and vascular function), understanding the contribution of age-related vestibular dysfunction, and how this interacts with these factors, is critical for informing interventions and treatments for balance problems and falls in older adults.
Closing the Gap on Health Disparities by Diversifying the Scientific Workforce: The importance of scientific workforce diversity, as an approach to reduce health disparities, has been recognized.[11] NIDCD is working to increase the diversity of scientists in the research pipeline in our scientific mission areas. For example, NIDCD participates in trans-NIH programs that promote diversity as well as health disparities research, including the F31 Diversity Fellowship, the Support of Competitive Research Program, the Maximizing Opportunities for Scientific and Academic Independent Careers Program, and the Diversity Supplement Program. In addition, NIDCD considers workforce diversity in R01 award decisions using a High Program Priority process to award R01 applications that fall outside of our automatic pay line. Further, we use R56 High Priority, Short Term Project awards to provide funds to allow the investigator to continue promising lines of research while revising and resubmitting an unsuccessful R01 grant application.
Despite these efforts, the number of funded investigators from diverse backgrounds who are focused on disorders in hearing, balance, taste, smell, voice, speech, and language remains discouragingly small. NIDCD plans to explore and implement new and renewed efforts to meet this need. We will aggressively disseminate information about the NIH Diversity Supplement Program and prioritize supplements for eligible faculty and post-doctoral fellows. NIDCD anticipates developing a two-day mentoring experience for candidates at various career stages who have submitted unsuccessful applications and developing new approaches/mechanisms to better support the training, mentoring, leadership, and engagement of underrepresented minority scientists. NIDCD leadership will identify ways to engage more effectively underrepresented minority populations in clinical research in our mission areas and ensure that our own workplace is diverse, respectful, and inclusive. NIDCD recognizes that for the United States to remain a global leader in scientific discovery and innovation, it is dependent upon a pool of highly talented scientists from diverse backgrounds.
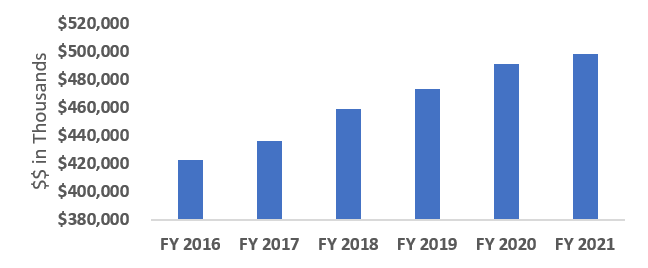
Overall Budget Policy: The FY 2022 President’s Budget request for NIDCD is $511.8 million, an increase of $13.7 million or 2.8 percent compared with the FY 2021 Enacted level.
IC Fact Sheet
History of NIDCD Research
For over 30 years, NIDCD research support has led to critical strides in uncovering knowledge in our research areas. NIDCD's focus is to bring national attention to normal and disordered processes of hearing, balance, taste, smell, voice, speech, and language and to contribute to advances in biomedical and behavioral research that will improve the lives of the tens of millions of people with these conditions. NIDCD employs a wide range of research approaches such as molecular genetics, biomedical imaging, cellular biology, nanotechnology, psychoacoustics, and structural and functional biology to advance the development of better diagnostics, prevention strategies, and treatment.

Debara Tucci, M.D., M.S., M.B.A., assumed her role as NIDCD Director in September 2019. She received her M.D. from the University of Virginia and practiced as a surgeon at Duke University for most of her career. At Duke, she co-founded the Duke Hearing Center and directed the cochlear implant program.
NIDCD Appropriations History
FY 2022 President's budget (PB) request is $511,792,000.
Facts and Figures
Data represent 3-year averages (FY 17-19)
- 132 Full Time Equivalent Employees
- 236 Principal Investigators
- 14% R01 Pay Line
- 206 Competing Research Project Grants
- 60 Competing Individual and Institutional Training Awards
- 27% Early-Stage-Investigator Success Rate (R01)
NIDCD Research Highlights
- Researchers use gene therapy in one of the first examples of successfully improving hearing and balance in mice.
- Scientists discover a link between common antidepressant drugs, which stimulate serotonin responses, and worsening tinnitus—a ringing in the ears when no sound is present.
- Researchers explore how expression of genes in stem cells in the tongue guide the development of taste cells.
- Scientists help explain hearing loss caused by cisplatin, a powerful and common drug used to treat many forms of cancer.
- Researchers develop brain-computer interface technology that allows people with loss of motor function to operate a computer or generate synthetic speech using just their thoughts.
- Scientists pinpoint genes tied to persistent stuttering. The findings provide new insights into the molecular underpinnings of the disorder and may contribute to the development of new diagnostic and therapeutic approaches for stuttering.
FY 2020 Spending by Mission Area
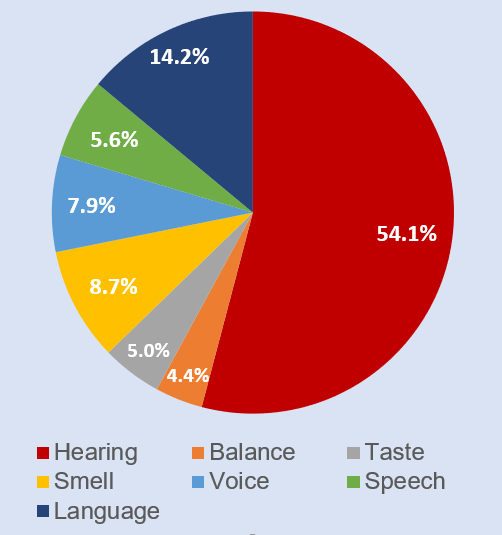
Current Activities
NIDCD prioritizes and supports initiatives that promote innovative research projects to improve accessible and affordable hearing health care, such as identifying barriers to care, assessing novel service delivery and screening models to improve the quality of life for adults with hearing loss.
Future Initiatives
NIDCD, with guidance of our advisory committees, will examine our current research portfolio and priorities, opportunities for growth and partnerships, and the impact on public health in developing the 2022-2026 NIDCD Strategic Plan with a vision toward the future. Our vision is that all Americans benefit from scientific discovery that informs effective and accessible prevention strategies and treatments for deafness and other communication disorders.
NIDCD will support research to determine the role and impact of digital health interventions (e.g., mobile health, telemedicine and telehealth, health information technology, wearable devices) to address secondary health effects of the social, behavioral, and economic changes following the COVID-19 pandemic, particularly among individuals who experience health disparities and other vulnerable populations, including those with hearing and communication impairments.
NIDCD Research Accomplishment: A Mind-Reading Computer for Those Who Cannot Speak
Augmentative and alternative communication (AAC) devices help people with voice, speech, or language disorders communicate. People with amyotrophic lateral sclerosis (or Lou Gehrig's disease) or brainstem stroke lose their ability to move their arms, legs, or body. They can become locked-in, where they are not able to speak, even though they are able to think and reason normally. However, with the advent of brain-computer interface (BCI) technology, which uses sensors to measure electrical signals in the brain to run a computer, NIDCD scientists believe they have found a way to restore hope to these individuals. In an important advance, NIDCD-supported engineers developed an approach to turn a person's thoughts directly into speech. The scientists trained a computer algorithm—that is typically used to interpret voice commands—to decipher brain activity instead. Individuals with epilepsy undergoing brain surgery listened to sentences spoken by different people. The researchers used the neural patterns measured from these patients to train the computer algorithm. When the patients simply thought of the words they had heard, the algorithm was able to accurately decipher the thoughts 75 percent of the time. NIDCD will continue to invest in assistive communication devices, such as BCI technology, to help people with amyotrophic lateral sclerosis, stroke, or neurodegenerative disease to regain the ability to communicate.
Major Changes in the Fiscal Year 2022 President's Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2022 President’s Budget for NIDCD, which is $13.7 million above FY 2021, for a total of $511.8 million.
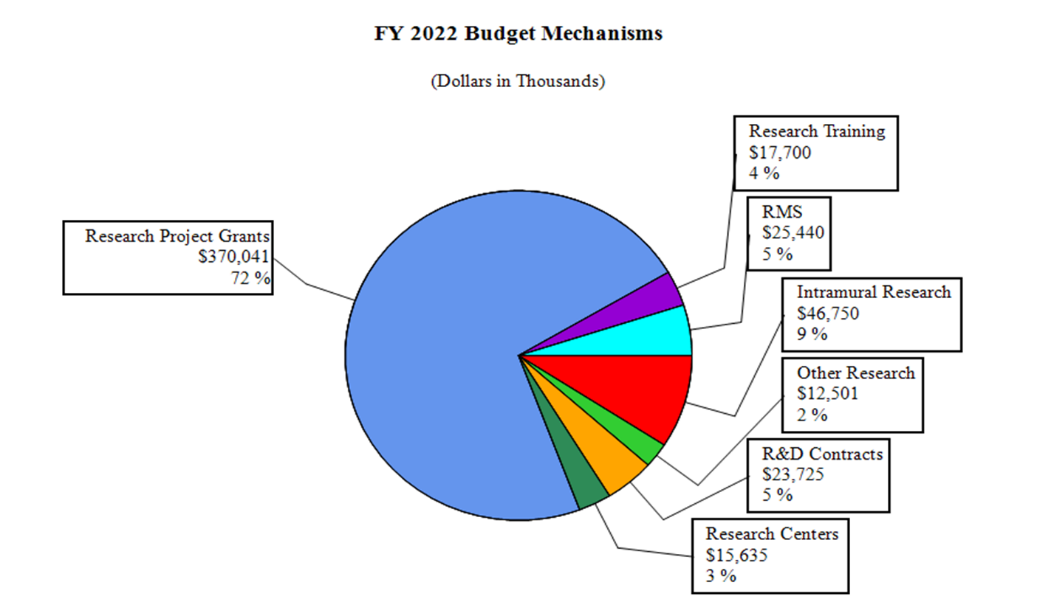
Research Project Grants (RPGs) (+$8.4 million; total $370.0 million):
NIDCD will fund 778 RPG awards in FY 2022, unchanged from the FY 2021 Enacted level. This includes 582 non-competing awards (a decrease of 8 awards and increase of $4.7 million from the FY 2021 Enacted level); and 170 competing RPGs (an increase of 8 awards and $4.0 million from the FY 2021 Enacted level).
Research Careers (+$0.5 million; total $9.8 million):
NIDCD will fund 58 Research Careers awards in FY 2022, an increase of 1 from the FY 2021 Enacted level.
Research Training Awards (+$0.8 million; total $17.7 million):
NIDCD will fund 316 Full-Time Training Positions (FTTPs) in FY 2022, an increase of 8 from the FY 2021 Enacted level.
Intramural Research (+$2.8 million; total $46.8 million):
In addition to planned inflationary growth in Intramural Research, in FY 2022 NIDCD will establish a new Neurotology Branch and lab which include three new tenure-track investigators.
| (Dollars in Thousands) | ||||||||
| MECHANISM | FY 2020 Final | FY 2021 Enacted | FY 2022 President's Budget | FY 2022 +/- FY 2021 Enacted | ||||
|---|---|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | No. | Amount | |
| Research Projects: | ||||||||
| Noncompeting | 622 | $264,153 | 590 | $263,470 | 582 | $268,120 | -8 | $4,650 |
| Administrative Supplements | (55) | $5,841 | (38) | $4,000 | (34) | $3,500 | (-4) | $-500 |
| Competing: | ||||||||
| Renewal | 28 | $15,264 | 28 | $15,500 | 30 | $16,350 | 2 | $850 |
| New | 149 | $59,966 | 134 | $63,000 | 140 | $66,191 | 6 | $3,191 |
| Supplements | 3 | $888 | 0 | $0 | 0 | $0 | 0 | $0 |
| Subtotal, Competing | 180 | $76,119 | 162 | $78,500 | 170 | $82,541 | 8 | $4,041 |
| Subtotal, RPGs | 802 | $346,113 | 752 | $345,970 | 752 | $354,161 | 0 | $8,191 |
| SBIR/STTR | 31 | $15,538 | 26 | $15,668 | 26 | $15,880 | 0 | $212 |
| Research Project Grants | 833 | $361,651 | 778 | $361,638 | 778 | $370,041 | 0 | $8,403 |
| Research Centers: | ||||||||
| Specialized/Comprehensive | 6 | $14,727 | 6 | $14,979 | 6 | $15,100 | 0 | $121 |
| Clinical Research | 0 | $501 | 0 | $535 | 0 | $535 | 0 | $0 |
| Biotechnology | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Comparative Medicine | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Research Centers in Minority Institutions | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Research Centers | 6 | $15,228 | 6 | $15,514 | 6 | $15,635 | 0 | $121 |
| Other Research: | ||||||||
| Research Careers | 51 | $8,196 | 57 | $9,332 | 58 | $9,825 | 1 | $493 |
| Cancer Education | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Cooperative Clinical Research | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Biomedical Research Support | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Minority Biomedical Research Support | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Other | 29 | $2,491 | 33 | $2,676 | 33 | $2,676 | 0 | $0 |
| Other Research | 80 | $10,687 | 90 | $12,008 | 91 | $12,501 | 1 | $493 |
| Total Research Grants | 919 | $387,566 | 874 | $389,160 | 875 | $398,177 | 1 | $9,017 |
| Ruth L Kirschstein Training Awards: | FTTPS | FTTPS | FTTPS | FTTPS | ||||
| Individual Awards | 133 | $6,494 | 150 | $8,079 | 155 | $8,550 | 5 | $471 |
| Institutional Awards | 158 | $8,307 | 158 | $8,788 | 161 | $9,150 | 3 | $362 |
| Total Research Training | 291 | $14,801 | 308 | $16,867 | 316 | $17,700 | 8 | $833 |
| Research & Develop. Contracts | 40 | $22,886 | 40 | $23,296 | 40 | $23,725 | 0 | $429 |
| (SBIR/STTR) (non-add) | (0) | ($160) | (0) | ($165) | (0) | ($170) | (0) | ($5) |
| Intramural Research | 59 | $42,763 | 65 | $44,000 | 65 | $46,750 | 0 | $2,750 |
| Res. Management & Support | 71 | $22,676 | 75 | $24,750 | 75 | $25,440 | 0 | $690 |
| SBIR Admin. (non-add) | (0) | ($0) | (0) | ($0) | (0) | ($0) | (0) | ($0) |
| Construction | $0 | $0 | $0 | $0 | ||||
| Buildings and Facilities | $0 | $0 | $0 | $0 | ||||
| Total, NIDCD | 130 | $490,692 | 140 | $498,073 | 140 | $511,792 | 0 | $13,719 |
| [1] All items in italics and brackets are non-add entries. | ||||||||
NATIONAL INSTITUTES OF HEALTH
NATIONAL INSTITUTE ON DEAFNESS AND OTHER COMMUNICATION DISORDERS
For carrying out section 301 and title IV of the PHS Act with respect to deafness and other communication disorders, [$498,076,000]$511,792,000.
| (Dollars in Thousands) | ||||||
| FY 2021 Enacted | $498,073 | |||||
| FY 2022 President's Budget | $511,792 | |||||
| Net change | $13,719 | |||||
| CHANGES | FY 2021 Enacted | FY 2022 President's Budget | Built-in Change from FY 2021 Enacted | |||
|---|---|---|---|---|---|---|
| FTEs | Budget Authority | FTEs | Budget Authority | FTEs | Budget Authority | |
| A. Built-in: | ||||||
| 1. Intramural Research: | ||||||
| a. Annualization of January 2021 pay increase & benefits | $12,571 | $13,753 | $34 | |||
| b. January FY 2022 pay increase & benefits | $12,571 | $13,753 | $343 | |||
| c. Paid days adjustment | $12,571 | $13,753 | $0 | |||
| d. Differences attributable to change in FTE | $12,571 | $13,753 | $0 | |||
| e. Payment for centrally furnished services | $7,744 | $8,131 | $387 | |||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | $23,685 | $24,866 | $644 | |||
| Subtotal | $1,409 | |||||
| 2. Research Management and Support: | ||||||
| a. Annualization of January 2021 pay increase & benefits | $14,292 | $14,724 | $38 | |||
| b. January FY 2022 pay increase & benefits | $14,292 | $14,724 | $394 | |||
| c. Paid days adjustment | $14,292 | $14,724 | $0 | |||
| d. Differences attributable to change in FTE | $14,292 | $14,724 | $0 | |||
| e. Payment for centrally furnished services | $2,349 | $2,466 | $117 | |||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | $8,109 | $8,250 | $324 | |||
| Subtotal | $873 | |||||
| Subtotal, Built-in | $2,282 | |||||
| CHANGES | FY 2021 Enacted | FY 2022 President's Budget | Program Change from FY 2021 Enacted | |||
|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | |
| B. Program | ||||||
| 1. Research Project Grants: | ||||||
| a. Noncompeting | 590 | $267,470 | 582 | $271,620 | -8 | $4,150 |
| b. Competing | 162 | $78,500 | 170 | $82,541 | 8 | $4,041 |
| c. SBIR/STTR | 26 | $15,668 | 26 | $15,880 | 0 | $212 |
| Subtotal, RPGs | 778 | $361,638 | 778 | $370,041 | 0 | $8,403 |
| 2. Research Centers | 6 | $15,514 | 6 | $15,635 | 0 | $121 |
| 3. Other Research | 90 | $12,008 | 91 | $12,501 | 1 | $493 |
| 4. Research Training | 308 | $16,867 | 316 | $17,700 | 8 | $833 |
| 5. Research and development contracts | 40 | $23,296 | 40 | $23,725 | 0 | $429 |
| Subtotal, Extramural | $429,323 | $439,602 | $10,279 | |||
| FTEs | FTEs | FTEs | ||||
| 6. Intramural Research | 65 | $44,000 | 65 | $46,750 | 0 | $1,341 |
| 7. Research Management and Support | 75 | $24,750 | 75 | $25,440 | 0 | $-183 |
| 8. Construction | $0 | $0 | $0 | |||
| 9. Buildings and Facilities | $0 | $0 | $0 | |||
| Subtotal, Program | 140 | $498,073 | 140 | $511,792 | 0 | $11,437 |
| Total built-in and program changes | $13,719 | |||||
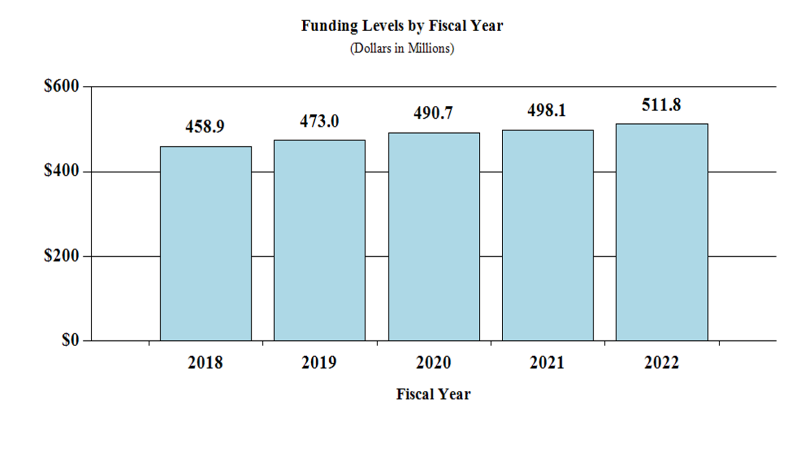
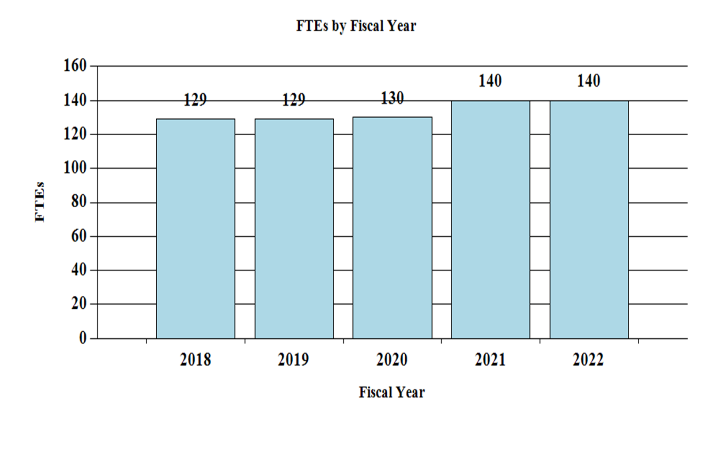
Fiscal Year 2022 Budget Graphs
History of Budget Authority and FTEs:
Distribution by Mechanism:
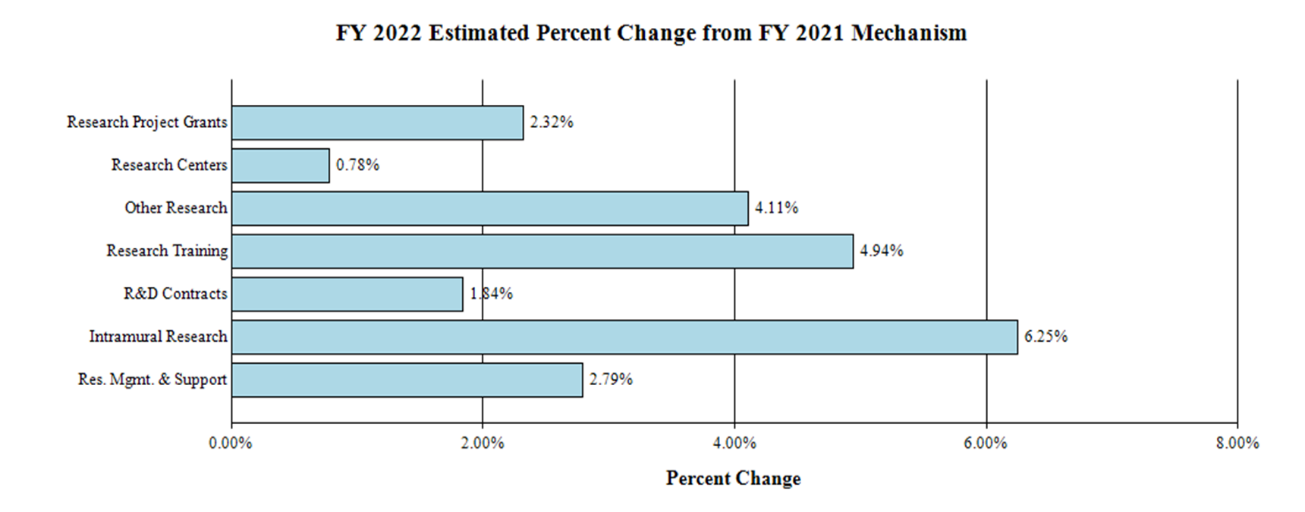
Change by Selected Mechanisms:
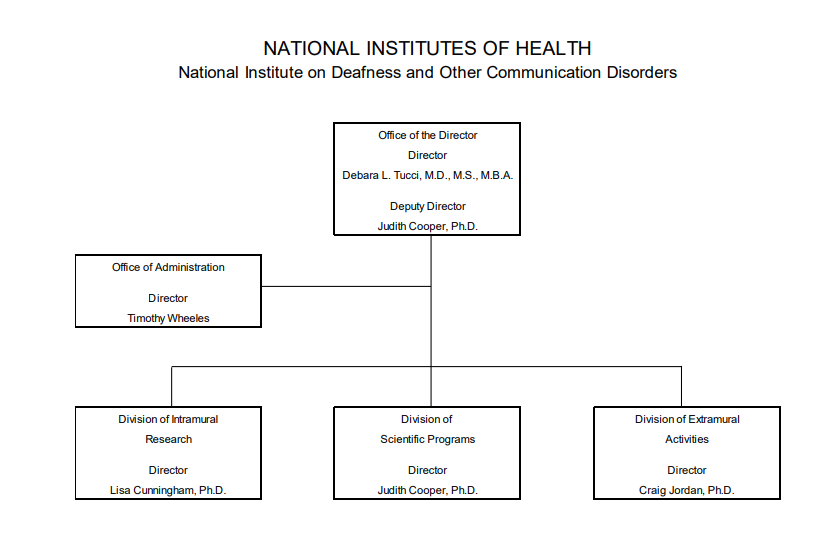
Organization Chart
| (Dollars in Thousands) | ||||||||
| FY 2020 Final | FY 2021 Enacted | FY 2022 President's Budget | FY 2022 +/- FY 2021 Enacted | |||||
|---|---|---|---|---|---|---|---|---|
| Extramural Research | FTE | Amount | FTE | Amount | FTE> | Amount | FTE | Amount |
| Detail | ||||||||
| Hearing and Balance | $238,038 | $240,316 | $246,070 | $5,754 | ||||
| Taste and Smell | $62,933 | $63,535 | $65,057 | $1,521 | ||||
| Voice, Speech, and Language | $124,282 | $125,472 | $128,476 | $3,004 | ||||
| Subtotal, Extramural | $425,253 | $429,323 | $439,602 | $10,279 | ||||
| Intramural Research | 59 | $42,763 | 65 | $44,000 | 65 | $46,750 | 0 | $2,750 |
| Research Management & Support | 71 | $22,676 | 75 | $24,750 | 75 | $25,440 | 0 | $690 |
| TOTAL | 130 | $490,692 | 140 | $498,073 | 140 | $511,792 | 0 | $13,719 |
| [1] Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | ||||||||
| FY 2020 Final | FY 2021 Enacted | FY 2022 President's Budget | FY 2022 +/- FY 2021 | |
|---|---|---|---|---|
| BA | $490,692,000 | $498,073,000 | $511,792,000 | +$13,719,000 |
| FTE | 130 | 140 | 140 | 0 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Program Descriptions
Hearing and Balance Program: Loss of hearing or balance imposes a significant social and economic impact upon individuals, their families, and the communities in which they live. About 2 out of every 1,000 children in the United States are born with a detectable level of hearing loss in one or both ears that can affect their speech, language, social, and cognitive development.[12] More than 1 in 20 American children have a problem with dizziness or imbalance.[13] Hearing and balance disorders cross all ethnic and socioeconomic lines. Among adults, hearing loss is the third most common chronic condition in the United States.[14] The prevalence of hearing loss increases with age, with nearly 25 percent of adults aged 65-74 having a disabling hearing loss.[15] This rate increases to 50 percent for those who are 75 and older.[12] This fact, along with the aging demographic in the United States, will increase the burden of age-related hearing loss in the years ahead. Accordingly, NIDCD supports research that will lead to improved treatments for, and prevention of, hearing and balance disorders across the lifespan. These research projects encompass over half of NIDCD’s grant portfolio.
One area of vigorous research is the potential use of gene therapy to correct mutated genes causing hearing and vestibular loss. For example, in 2019, the hearing research community ended a 40-year quest to identify the key signaling receptor for hearing – the TMC1 gene and a closely related gene called TMC2 which is also important for hearing. Mutations in the TMC genes are known to cause deafness and vestibular deficits in humans and mice. Now, NIDCD-funded researchers are capitalizing on the finding by developing gene therapies that target the TMC1 gene to restore hearing and balance in animal models. A research team, funded in part by NIDCD, used novel, synthetic viral vectors to deliver the correct TMC1 gene code into mice that had hearing and balance impairments because of mutations in the TMC1 and TMC2 genes.[16] This approach was successful in inserting correct copies of TMC1 genes into inner ear sensory cells. The scientists found that restoring TMC1 function in those mice soon after birth restored sensory cell function and survival, hearing and balance function, and neural activation in the brain to hearing and balance stimulation. Although challenges remain, the findings suggest that TMC1 gene therapy merits further development and eventual translation into human clinical trials.
For many individuals with severe to profound hearing loss, cochlear implants vastly improve their ability to communicate by bypassing the damaged part of the auditory system. Cochlear implants are comprised of a series of electrodes in an array that is inserted directly into the cochlea (the snail-shaped structure in the inner ear) to stimulate the auditory nerve, which then transmits sound information to the brain. After cochlear implant surgery, an audiologist needs to “fit” the cochlear implant to determine the stimulation level of each electrode based on perceived loudness, and to assign a frequency to an electrode through trial and error to determine its position within the cochlea. This process needs to be performed for up to 12 or more electrodes within the electrode array and is very time-consuming. NIDCD is funding a number of grants that support development and assessment of personalized cochlear implant settings and placement.
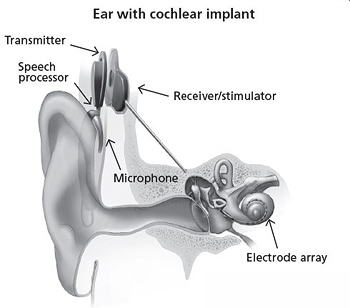
NIDCD is funding researchers who are developing an image-guided cochlear implant programming (IGCIP) paradigm to assist audiologists with the cochlear implant fitting process. Recently, these researchers developed a novel automated process which takes a clinical computed tomography (CT) image and determines the electrodes’ locations for a number of different cochlear implant brands.[17] Electrode location is critically important for the IGCIP process, and this novel method outperformed the current state of the art cochlear implant electrode localization methods. NIDCD is also funding another research team to explore IGCIP for children 6 to 12 years old receiving cochlear implants to see if the individualized fitting strategy results in improvements in auditory function, speech recognition, and reading. Currently, cochlear implant outcomes for children are highly variable. The research team is employing a double-blind randomized design, and the outcomes will be compared to the current standard of care, which has immediate translational implications for the management of these children. Finally, NIDCD is supporting a small business grant that will enable new technology for manufacturing cochlear implant electrodes that have a smaller array size for residual hearing preservation, which can include drug delivery channels to promote inner ear health, and have gauges to detect stress (internal resisting forces from the cochlea) and strain (displacement and deformation of the electrode) for improved performance during surgical insertion.
NIDCD supports a research program to understand normal and disordered balance and vestibular (inner ear balance) systems. Balance disorders affect a large proportion of the population, particularly the elderly. According to the Centers for Disease Control and Prevention, the estimated medical cost of falls across the U.S. healthcare system is $50 billion annually.[18] This includes $38 billion paid by Medicare and Medicaid and $12 billion paid by private and other payers. As mentioned in the Director’s Overview, NIDCD in conjunction with NIA has announced a new initiative to understand how the nervous system regulates balance in older adults. Significant gaps exist in the current research literature on how aging impacts the vestibular system, and this information is critical for developing interventions and treatments for balance problems and falls in older adults. NIDCD already supports several grants focused on improving diagnosis and assessment of balance impairment in older adults. One small business grant aims to develop a mobile app for vestibular rehabilitation for older adults with dizziness. Another research team has developed a machine learning approach—computer algorithms that improve automatically with experience—to evaluate balance during in-home training.[19] Typically, in-home balance training is not as effective compared to in-clinic training. Using a machine learning approach to evaluate balance training and provide interactive feedback helps assess treatments, suggest therapies, and predict who may be more at risk for falls. This approach could also improve user compliance outside a clinical setting and serve to reduce falls for this vulnerable population.
Budget Policy: The FY 2022 President’s Budget request for the hearing and balance program is $246.1 million, an increase of $5.8 million or 2.4 percent compared to the FY 2021 Enacted level.
Answering the Call: Accessible and Affordable Hearing Health Care
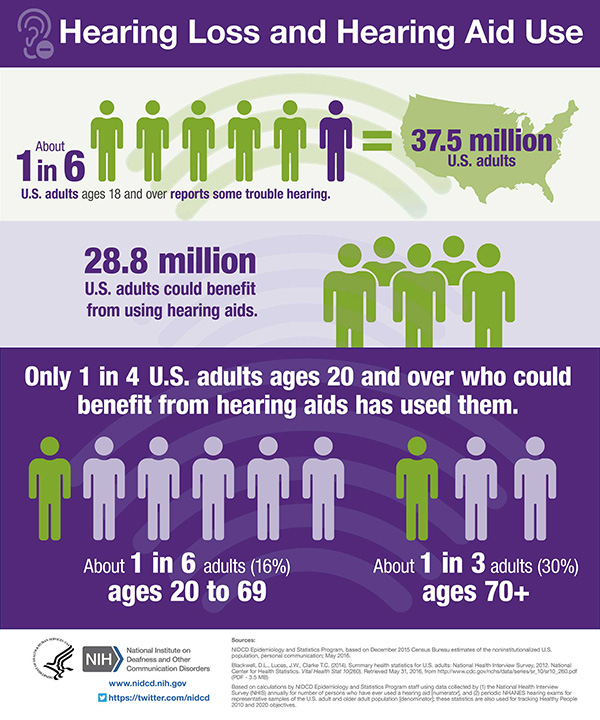
Untreated hearing loss may lead to serious conditions such as depression, anxiety, dementia, reduced mobility, and falls. Hearing aids and other assistive devices can significantly improve the quality of life for many people, but only 1 in 4 adults who could benefit from hearing aids has ever used them. As the lead federal agency supporting research to prevent, detect, and treat hearing loss, NIDCD has made research on improving access to and affordability of hearing health care a priority over the past decade.
Since 2009, NIDCD has published a dozen funding opportunities leading to support of 40 research projects on the topic. In 2016, the National Academies of Sciences, Engineering, and Medicine published a consensus report, Hearing Health Care for Adults: Priorities for Improving Access and Affordability. NIDCD co-sponsored this effort with other federal agencies and the Hearing Loss Association of America. Independent expert panelists encouraged agencies, organizations, and professionals to improve access and affordability of services, develop innovative technologies, and better inform consumers about their choices for managing hearing loss. One of the panel’s recommendations was for the U.S. Food and Drug Administration (FDA) to create and regulate a new category of over-the-counter (OTC) hearing devices. In 2017, a new law directed the FDA to establish guidelines and quality standards for this new category of OTC hearing aids. A draft of these regulations is underway and expected to be released in 2021.
NIDCD-funded research informed the expert panel’s recommendations as well as the policy changes around improving adult hearing health care. The research shows that people with hearing loss are able to independently adjust the settings on their hearing devices for different listening environments and are generally more satisfied with the sound of the devices than those fit by an audiologist. An NIDCD-funded clinical trial comparing an OTC delivery model of hearing aids with traditional audiologist-fit devices found that hearing aid users in both groups reported similar benefits. The results support the viability of a direct-to-consumer service delivery model. NIDCD also supports research that improves hearing aid technology. One small-business research grant funded by NIDCD led to the first self-fitting hearing aid approved by the FDA.
NIDCD remains committed to improving the landscape of adult hearing health care through research to fill remaining scientific and clinical gaps.
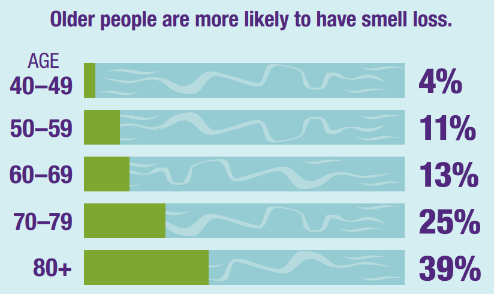
Taste and Smell Program: Taste and smell disorders are underreported, and often undiagnosed. NIDCD supports studies of the chemical senses known as taste, smell, and chemesthesis (chemically provoked irritation) to enhance our understanding of how individuals gather information about their environment and how human chemosensory disorders can be diagnosed and treated. With age, the olfactory (sense of smell) system is less able to repair and regenerate, and our sense of smell declines. In U.S. adults over age 40, smell tests show that about 1 out of 8 people has some smell loss, and about 1 out of 30 people has very little or no sense of smell.[20] Since both taste and smell contribute to flavor perception, such olfactory deficits affect the flavor of foods and consequently negatively affect food intake, diet, overall nutrition, and health status. NIDCD encourages further studies of age-related decline in olfactory sensitivity including the development of better diagnostic tests to assess chemosensory loss and animal models to study why this decline occurs and how to prevent or treat it.
Loss of the sense of smell, also called anosmia, can be an early sign of neurological disorders, including both Alzheimer’s disease and Parkinson’s disease. Anosmia is also a common—and usually early—symptom of COVID-19. In published reports, SARS CoV-2-infected individuals develop anosmia anywhere from 50 to almost 100 percent of the time. NIDCD-supported research teams are examining olfactory tissue from individuals who died of COVID-19 to look at which cells are actively infected with SARS CoV-2 and identify potential COVID-related cell death and changes in blood vessels, immune responses, or gene expression. NIDCD is also supporting research through the RADx-rad initiative to develop inexpensive and objective tests to screen for COVID-19 and to determine whether smell loss can predict disease severity or other neurological consequences of COVID-19 disease.
NIDCD-funded researchers have sought to understand how the brain can decipher the myriads of odors present in the environment given the finite number of olfactory receptors in the nose. The olfactory epithelium—the part of the nervous system that directly perceives smell—is located deep within the nose and is made up of many thousands of nerve cells that each contain a single olfactory receptor type that reacts to a specific odor. Studies using simple odors suggest that when we smell something, a specific combination of these nerve cells becomes activated, forming a code that is interpreted by the brain as that particular odor. In the past, researchers were only able to study a limited fraction of the olfactory epithelium, and the methods they used often damaged the tissue, making it hard to draw definitive conclusions. Now, an ultra-fast, 3D imaging technique called Swept, Confocally-Aligned Planar Excitation (SCAPE)microscopy, developed through the NIH Brain Research through Advancing Innovative Technologies (BRAIN) Initiative, allows a greater volume of tissue to be viewed in a way that is much less damaging to delicate networks of living cells. NIDCD scientists in collaboration with other NIH-funded researchers used SCAPE to watch how the mouse olfactory epithelium reacted in real time to complex odors.[21] They found that the information that the nose sends to the brain about mixture of odors is more than the sum of its parts. They found that in some cases an odor can enhance a neuron’s response to a first odor, or in other cases, it can dampen the neuron’s response. These findings are transformative for how olfactory researchers understand odor/odor receptor interaction. Being able to change the way a receptor responds to one substance could be very important for drug development. Further, the findings from the olfactory epithelium could translate into possible ways to manipulate responses of cell types that might be involved in other diseases.
Budget Policy: The FY 2022 President’s Budget request for the taste and smell program is $65.1 million, an increase of $1.5 million or 2.4 percent compared to the FY 2021 Enacted level.
Voice, Speech, and Language Program:Disorders involving voice, speech, or language can have an overwhelming effect on an individual’s health and quality of life. These disorders affect people of all ages with or without hearing impairment, such as children with autism, people who stutter, and adults with acquired communication disorders. Voice, speech, and language disorders also come at a significant cost. The cost to society of laryngeal disorders is estimated at $11 billion annually due to work-related disability, lost productivity, and direct health care cost.[22] Further, nearly eight percent of children ages 3-17 years have had a communication disorder during the past 12 months, according to data from the National Health Interview Survey, 2012.[23] By the first grade, roughly five percent of children have noticeable speech disorders.[24] In children, delayed speech and language acquisition or impairment are very often significant predictors of future academic, social, vocational, and adaptive outcomes.[25]
Teachers, politicians, healthcare workers, singers, and public speakers are among those who make great demands on their voices as a necessary part of their work. This puts them at risk for developing voice problems. An estimated 17.9 million adults in the United States report problems with their voice each year.[26] NIDCD supports basic research to understand diseases and stresses that can harm the voice, and clinical research to test new ways to diagnose, treat, or cure voice disorders. NIDCD-funded researchers are working to identify neural, biological, and behavioral factors that put certain individuals at risk for voice disorders. The scientists are employing imaging techniques, measuring stress hormones, and identifying behaviors in early career teachers with vocal fatigue compared to control participants. They hope to make fundamental discoveries to help clinicians understand the role of stress in voice disorders caused by muscle tension and to identify risk factors to recognize those who may develop voice disorders. The knowledge will help design preventative measures and optimize the long-term success of interventions for a broad range of behavioral voice and speech disorders.
Roughly three million Americans stutter. Approximately 5 to 10 percent of all children will stutter for some period in their life, lasting from a few weeks to several years. The majority of children recover from stuttering, but for the remaining 25 percent who continue to stutter, stuttering can persist as a lifelong and sometimes debilitating communication disorder. NIDCD scientists have identified variants in four genes that account for some cases of stuttering in many populations around the world, including the United States and Europe. All of these genes encode proteins that direct traffic within cells, ensuring that various cell components get to their proper location within the cell. Such deficits in cellular trafficking are a newly recognized cause of many neurological disorders. Researchers are now studying how this defect in cellular trafficking leads to specific deficits in speech fluency. In a recent advance, NIDCD-funded researchers identified the large-scale cortical network that characterizes stuttering, using functional connectivity MRI and mathematical models to determine connections between brain areas and stuttering gene expression.[27] They found that the location of one of the stuttering genes in the brain highly coincides with the neural network involved in stuttering. These results provide the first evidence of possible causal links between gene mutations and aberrant brain connectivity in humans who stutter. Researchers could one day develop effective treatments if they can identify targets within the aberrant neural mechanisms that result in persistent developmental stuttering.

Language acquisition is the process by which children acquire the ability to perceive and understand language, as well as the ability to produce and use words and sentences to communicate. Developmental language disorder (DLD) is a communication disorder that interferes with the development of language skills in children who have no hearing loss or intellectual disabilities. It is one of the most common developmental disorders, affecting approximately seven percent of children in kindergarten.[28] The impact on society is felt when these children require expenditure of special education dollars, experience school failure, and face limited job prospects. NIDCD supports a wide variety of research on the causes, symptoms, diagnosis, and treatment of DLD. For example, NIDCD is supporting several studies to develop and improve early treatments for DLD, which benefit young children. One research team is focused on understanding how socio-economic disparities put children from low-income families at risk for DLD. They are also studying the effects of COVID-19 and related stressors on caregiver-infant interactions and short-term language growth among infants in low-income families. In another study, researchers are conducting a Phase II clinical trial that evaluates the efficacy of an 18-month parent-implemented intervention for accelerating language growth and reducing risk for DLD in preschool children with language delays. NIDCD is also supporting studies to determine the optimal word learning parameters (e.g., words per session, frequency of sessions) to maximize language acquisition in toddlers, preschoolers, and kindergarteners with language impairments.
Budget Policy: The FY 2022 President’s Budget request for the voice, speech, and language program is $128.5 million, an increase of $3.0 million or 2.4 percent compared with the FY 2021 Enacted level.
Capitalizing on Foundational Investments and Beyond: Visualizing the Human Inner Ear
The delicate structures of the human inner ear are surrounded by the dense temporal bone, which serves as protective armor. This anatomical location hinders the ability of clinicians and scientists to visualize the cellular components of the auditory and vestibular systems at sufficient resolution to allow for detailed diagnosis and treatment of many causes of auditory and vestibular dysfunction. Advanced imaging of the inner ear could provide critically important information needed to devise optimal treatment strategies for those with hearing and balance impairments, including a determination of candidacy for hearing restoration therapies in clinical trials. Presently, we do not have techniques with high enough resolution for structural imaging—let alone functional imaging—of the living human inner ear.
Current clinical imaging techniques of the living human inner ear are generally limited to standard computerized tomography without contrast or magnetic resonance imaging with contrast. Improved imaging of the human inner ear is a critical unmet need that could directly impact patient care. To meet this need, NIDCD convened intramural and extramural scientists for a workshop in 2019, Visualizing the Human Inner Ear. This workshop convened auditory, vestibular, and imaging experts to discuss ways to image living human inner ear tissue at high resolution across both structural and functional levels to advance the ability to diagnose and treat auditory and vestibular deficits.
Visualization of postmortem human temporal bone specimens is possible, but specimens are laborious to retrieve and prepare for study. In response to this need, workshop participants also discussed the importance of postmortem human temporal bone studies and resources for translating laboratory-based hearing science studies to clinical treatment in humans. It is important to confirm that the hypotheses generated from animal models hold true in humans before beginning to consider gene therapy or other treatments. Access to human auditory and vestibular tissue with known medical histories is an important tool to be able to make that translational step.
Detailed images of the structure and functioning of the living human inner ear would greatly advance NIDCD’s mission to help prevent, detect, diagnose, and treat deafness, balance, and other communication disorders. To date, such images have not been possible, but technology is rapidly evolving, putting a once lofty goal within reach.
Intramural Research Program (IRP): The NIDCD Intramural Research Program conducts basic and clinical research in human communication, with a primary focus on hearing and balance.
One NIDCD lab is working to prevent hearing loss caused by cisplatin—a life-saving cancer drug. While cisplatin is rapidly cleared from the body, it builds up in the inner ear and kills the sensory hair cells that detect sound. Once lost, auditory hair cells in mammals are not spontaneously repaired or replaced, resulting in hearing loss. Previous research from this team showed that protective molecules, called heat shock proteins, helped protect hair cells under conditions of stress. U.S. Food and Drug Administration (FDA)-approved statin medications, taken by more than 35 million Americans to lower cholesterol, also boost cellular levels of heat shock proteins. Thus, the team wanted to see if statins could preserve hearing in mice treated with cisplatin therapy. They found that mice treated with cisplatin and statins had better hearing and lost fewer hair cells than mice treated with cisplatin alone.[29] Now the researchers have begun to extend these findings to humans by examining hearing function in people treated with cisplatin who are also taking a statin drug. Statins are inexpensive, well-tolerated, and do not reduce the therapeutic efficacy of cisplatin. The team hopes that if studies show statins reduce cisplatin-induced hearing loss in humans, doctors can rapidly translate the research into treatment to preserve hearing in many individuals being treated for cancer.
NIDCD intramural scientists are also working to improve cancer treatments, particularly in head and neck cancers that have resisted standard treatments. The human immune system is designed to naturally attack and destroy cancer cells, but cancers avoid these killer cells by suppressing the immune response inside and outside the tumor. NIDCD intramural scientists along with those from the National Cancer Institute collaborated to reprogram a type of tumor-killing cell found in the body, called natural killer cells, to be more effective in fighting cancer. These new reprogrammed cells are called high affinity natural killer cells, or haNKs. The haNKs not only kill cancer cells but also overcome the immunosuppression found inside and outside tumors.[30] When the research team tested their haNK cells on mouse head and neck tumors, the treatment eliminated or slowed tumor growth. The team then tested their haNKs on human tumor cells in culture. The reprogrammed haNKs killed human cancer cells and overcame the immunosuppression in the blood from individuals with advanced head and neck cancer. Now they plan to take this therapy into the clinic to test its safety and to determine whether it can enhance treatment response for individuals with cancer who haven’t responded to other immunotherapies.
Intramural scientists at NIDCD and their collaborators developed a detailed chronology of genes expressed in the cochlea. The team analyzed data from 30,000 mouse cochlea cells at three different developmental time points, to identify what genes are switched on at different times to drive cells to adopt one of the possible cell identities within the mature cochlea.[31] They describe genes important for driving cells towards supporting cell identities, as well as genes that drive a cell to adopt sensory hair cell identity. This knowledge may help researchers understand how to turn on these same genes in human stem cells. If scientists can learn to produce hair cells from stem cells, they may be able to test cell replacement approaches for reversing some forms of hearing loss. Further, the researchers shared their data through a web-based “gene Expression Analysis Resource” portal. Now, other scientists may use the gene expression patterns the team describes to understand what goes wrong in some forms of inherited deafness.
Budget Policy: The FY 2022 President’s Budget request for the intramural research program is $46.8 million, an increase of $2.8 million or 6.3 percent compared with the FY 2021 Enacted level.
Research Management and Support (RMS) Program: NIDCD RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS functions also include strategic planning, coordination, and evaluation of the Institute’s programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. The Institute currently supports approximately 1,400 research grants, training awards, and Research & Development contracts.
With COVID-19 restriction preventing large, in-person seminars from occurring, NIDCD continues a long-standing tradition to educate the public and inform the scientific community about research related to deafness and other communication disorders by hosting several virtual events. Designed for administrative and support staff as well as scientists, NIDCD's Beyond the Lab, Understanding Communication Disorders speaker series gives attendees the opportunity to learn about NIDCD’s research and about scientific advances in communication disorders—conditions that directly affect about one in six Americans during their lifetime. The series is open to the larger NIH community and to the public. Another outreach event, EARssentials: Concepts and Techniques of Contemporary Hearing Research is a week-long course that provides students with an overall conceptual view of the auditory system as well as exposure to laboratory techniques unique to research on the inner ear. This course is hosted and taught primarily by scientists from NIDCD’s intramural research program. This year, this course was successfully converted to an all-virtual presentation, which opened participation to a wider audience of participants across the country. The primary audience for this course is new intramural NIDCD trainees, including postdoctoral, graduate, postbaccalaureate, and summer students. Scientists from other institutions also attend. All attendees gain exposure to the scientific basis of NIDCD research, and the speaker series and course serves as gateways for public stakeholders who have an interest in our mission areas.
To provide effective and efficient administrative management services, maintain good stewardship of resources, and mitigate risk management through employee engagement, the Institute established an Administrative Strategic Plan (ASP). The plan provides employees with new tools to improve administering and managing services in support of NIDCD's mission and to improve the quality of work life for NIDCD staff. Examples of ASP tools are featured in a series of presentations, one-page documents, and policies and procedures that enable all NIDCD staff to learn about professional development/training, human resources processes/services, risk awareness, trans-NIDCD/agency/department opportunities, and Optimize NIH: Increase Administrative Efficiency. In addition to improving administrative services, the ASP provides important opportunities to develop leadership and team-building competencies as each tool is developed based on teams of NIDCD staff.
Budget Policy: The FY 2022 President’s Budget request for RMS is $25.4 million, an increase of $0.7 million or 2.8 percent compared with the FY 2021 Enacted level.
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation |
|---|---|---|---|---|
| 2013 | $417,297,000 | $418,562,000 | $416,272,755 | |
| Rescission | $832,546 | |||
| Sequestration | ($20,894,030) | |||
| 2014 | $422,936,000 | $420,125,000 | $404,049,000 | |
| Rescission | $0 | |||
| 2015 | $403,933,000 | $405,302,000 | ||
| Rescission | $0 | |||
| 2016 | $416,241,000 | $412,366,000 | $424,860,000 | $423,031,000 |
| Rescission | $0 | |||
| 2017[1] | $422,936,000 | $434,126,000 | $441,778,000 | $436,875,000 |
| Rescission | $0 | |||
| 2018 | $325,846,000 | $443,624,000 | $451,768,000 | $459,974,000 |
| Rescission | $0 | |||
| 2019 | $423,992,000 | $465,467,000 | $474,653,000 | $474,404,000 |
| Rescission | $0 | |||
| 2020 | $408,358,000 | $497,590,000 | $500,270,000 | $490,692,000 |
| Rescission | $0 | |||
| 2021 | $446,397,000 | $494,912,000 | $506,670,000 | $498,076,000 |
| Rescission | $0 | |||
| 2022 | $511,792,000 | |||
| [1]Budget Estimate to Congress includes mandatory financing. | ||||
Authorizing Legislation
| (Dollars in Thousands) | |||
| Source of Funding | FY 2020 Final |
FY 2021 Enacted |
FY 2022 President's Budget |
|---|---|---|---|
| Appropriation | $490,692 | $498,076 | $511,792 |
| Secretary's Transfer | 0 | 0 | 0 |
| OAR HIV/AIDS Transfers | 0 | -3 | 0 |
| Subtotal, adjusted budget authority | $490,692 | $498,073 | $511,792 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | $490,692 | $498,073 | $511,792 |
| Unobligated balance lapsing | -5 | 0 | 0 |
| Total obligations | $490,687 | $498,073 | $511,792 |
| [1] Excludes the following amounts (in thousands) for reimbursable activities carried out by this account: FY 2020 - $349FY 2021 - $1,000FY 2022 - $1,000 |
|||
| (Dollars in Thousands) | ||||
| FY 2021 Enacted | FY 2022 President's Budget | FY 2022 +/- FY 2021 Enacted | ||
|---|---|---|---|---|
| Total Compensable workyears: | ||||
| Full-time equivalent | 140 | 140 | 0 | |
| Full-time equivalent of overtime and holiday hours | 0 | 0 | 0 | |
| Average ES salary | $199 | $205 | $5 | |
| Average GM/GS grade | 12.6 | 12.6 | 0.0 | |
| Average GM/GS salary | $123 | $126 | $3 | |
| Average salary, Commissioned Corps (42 U.S.C. 207) | $138 | $142 | $4 | |
| Average salary of ungraded positions | $124 | $127 | $3 | |
| OBJECT CLASSES | FY 2021 Enacted | FY 2022 President's Budget | FY 2022 +/- FY 2021 | |
| Personal Compensation | ||||
| 11.1 | Full-time Permanent | 13,198 | 13,898 | 700 |
| 11.3 | Other Than Full-time Permanent | 5,016 | 5,281 | 264 |
| 11.5 | Other Personnel Compensation | 584 | 597 | 13 |
| 11.7 | Military Personnel | 137 | 140 | 4 |
| 11.8 | Special Personnel Services payments | 1,384 | 1,519 | 134 |
| 11.9 | Subtotal Personnel Compensation | $20,319 | $21,435 | $1,116 |
| 12.1 | Civilian Personnel Benefits | 6,482 | 6,979 | 496 |
| 12.2 | Military Personnel Benefits | 62 | 63 | 2 |
| 13.0 | Benefits to Former Personnel | 0 | 0 | 0 |
| Subtotal Pay Costs | $26,864 | $28,477 | $1,614 | |
| 21.0 | Travel & Transportation of Persons | 222 | 128 | -93 |
| 22.0 | Transportation of Things | 38 | 41 | 3 |
| 23.1 | Rental Payments to GSA | 0 | 0 | 0 |
| 23.2 | Rental Payments to Others | 0 | 0 | 0 |
| 23.3 | Communications, Utilities & Misc. Charges | 102 | 106 | 4 |
| 24.0 | Printing & Reproduction | 0 | 0 | 0 |
| 25.1 | Consulting Services | 11,188 | 11,738 | 549 |
| 25.2 | Other Services | 5,285 | 5,420 | 135 |
| 25.3 | Purchases of goods and services from government accounts | 39,168 | 40,533 | 1,365 |
| 25.4 | Operation & Maintenance of Facilities | 5 | 5 | 0 |
| 25.5 | R&D Contracts | 3,643 | 3,642 | -1 |
| 25.6 | Medical Care | 163 | 170 | 7 |
| 25.7 | Operation & Maintenance of Equipment | 1,245 | 1,253 | 7 |
| 25.8 | Subsistence & Support of Persons | 0 | 0 | 0 |
| 25.0 | Subtotal Other Contractual Services | $60,697 | $62,760 | $2,063 |
| 26.0 | Supplies & Materials | 2,047 | 2,104 | 57 |
| 31.0 | Equipment | 2,072 | 2,294 | 222 |
| 32.0 | Land and Structures | 5 | 5 | 0 |
| 33.0 | Investments & Loans | 0 | 0 | 0 |
| 41.0 | Grants, Subsidies & Contributions | 406,027 | 415,877 | 9,850 |
| 42.0 | Insurance Claims & Indemnities | 0 | 0 | 0 |
| 43.0 | Interest & Dividends | 0 | 0 | 0 |
| 44.0 | Refunds | 0 | 0 | 0 |
| Subtotal Non-Pay Costs | $471,209 | $483,315 | $12,105 | |
| Total Budget Authority by Object Class | $498,073 | $511,792 | $13,719 | |
| [1] Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | ||||
| (Dollars in Thousands) | |||
| OBJECT CLASSES | FY 2021 Enacted | FY 2022 President's Budget | FY 2022 +/- FY 2021 |
|---|---|---|---|
| Personnel Compensation | |||
| Full-Time Permanent (11.1) | $13,198 | $13,898 | $700 |
| Other Than Full-Time Permanent (11.3) | $5,016 | $5,281 | $264 |
| Other Personnel Compensation (11.5) | $584 | $597 | $13 |
| Military Personnel (11.7) | $137 | $140 | $4 |
| Special Personnel Services Payments (11.8) | $1,384 | $1,519 | $134 |
| Subtotal Personnel Compensation (11.9) | $20,319 | $21,435 | $1,116 |
| Civilian Personnel Benefits (12.1) | $6,482 | $6,979 | $496 |
| Military Personnel Benefits (12.2) | $62 | $63 | $2 |
| Benefits for Former Personnel (13.0) | $0 | $0 | $0 |
| Subtotal Pay Costs | $26,864 | $28,477 | $1,614 |
| Travel & Transportation of Persons (21.0) | $222 | $128 | -$93 |
| Transportation of Things (22.0) | $38 | $41 | $3 |
| Rental Payments to Others (23.2) | $0 | $0 | $0 |
| Communications, Utilities & Misc. Charges (23.3) | $102 | $106 | $4 |
| Printing & Reproduction (24.0) | $0 | $0 | $0 |
| Other Contractual Services: | |||
| Consultant Services (25.1) | $11,188 | $11,738 | $549 |
| Other Services (25.2) | $5,285 | $5,420 | $135 |
| Purchases from government accounts (25.3) | $22,983 | $25,340 | $2,357 |
| Operation & Maintenance of Facilities (25.4) | $5 | $5 | $0 |
| Operation & Maintenance of Equipment (25.7) | $1,245 | $1,253 | $7 |
| Subsistence & Support of Persons (25.8) | $0 | $0 | $0 |
| Subtotal Other Contractual Services | $40,707 | $43,755 | $3,049 |
| Supplies & Materials (26.0) | $2,047 | $2,104 | $57 |
| Subtotal Non-Pay Costs | $43,116 | $46,134 | $3,019 |
| Total Administrative Costs | $69,979 | $74,611 | $4,632 |
| OFFICE/DIVISION | FY 2020 Final | FY 2021 Enacted | FY 2022 President's Budget | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Division of Extramural Activities | |||||||||
| Direct: | 19 | - | 19 | 19 | - | 19 | 19 | - | 19 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 19 | - | 19 | 19 | - | 19 | 19 | - | 19 |
| Division of Intramural Activities | |||||||||
| Direct: | 58 | 1 | 59 | 64 | 1 | 65 | 64 | 1 | 65 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 58 | 1 | 59 | 64 | 1 | 65 | 64 | 1 | 65 |
| Division of Scientific Programs | |||||||||
| Direct: | 15 | - | 15 | 17 | - | 17 | 17 | - | 17 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 15 | - | 15 | 17 | - | 17 | 17 | - | 17 |
| Office of Administration | |||||||||
| Direct: | 35 | - | 35 | 37 | - | 37 | 37 | - | 37 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 35 | - | 35 | 37 | - | 37 | 37 | - | 37 |
| Office of the Director | |||||||||
| Direct: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Total: | 129 | 1 | 130 | 139 | 1 | 140 | 139 | 1 | 140 |
| Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||||||||
| FTEs supported by funds from Cooperative Research and Development Agreements. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FISCAL YEAR | Average GS Grade | ||||||||
| 2018 | 12.4 | ||||||||
| 2019 | 12.4 | ||||||||
| 2020 | 12.4 | ||||||||
| 2021 | 12.6 | ||||||||
| 2022 | 12.6 | ||||||||
| GRADE | FY 2020 Final | FY 2021 Enacted | FY 2022 President's Budget |
|---|---|---|---|
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | $197,300 | $199,300 | $204,681 |
| General Schedule | |||
| GM/GS-15 | 22 | 27 | 27 |
| GM/GS-14 | 21 | 19 | 19 |
| GM/GS-13 | 20 | 27 | 27 |
| GS-12 | 19 | 18 | 18 |
| GS-11 | 5 | 4 | 4 |
| GS-10 | 0 | 0 | 0 |
| GS-9 | 8 | 8 | 8 |
| GS-8 | 5 | 5 | 5 |
| GS-7 | 2 | 2 | 2 |
| GS-6 | 0 | 0 | 0 |
| GS-5 | 1 | 1 | 1 |
| GS-4 | 0 | 0 | 0 |
| GS-3 | 1 | 0 | 0 |
| GS-2 | 1 | 1 | 1 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 105 | 112 | 112 |
| Commissioned Corps (42 U.S.C. 207) | |||
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 1 | 1 | 1 |
| Senior Grade | 0 | 0 | 0 |
| Full Grade | 0 | 0 | 0 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 1 | 1 | 1 |
| Ungraded | 40 | 43 | 43 |
| Total permanent positions | 105 | 112 | 112 |
| Total positions, end of year | 147 | 157 | 157 |
| Total full-time equivalent (FTE) employment, end of year | 130 | 140 | 140 |
| Average ES salary | $197,300 | $199,300 | $204,681 |
| Average GM/GS Grade | 12.4 | 12.6 | 12.6 |
| Average GM/GS salary | $121,376 | $122,590 | $125,900 |
| [1] Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||