- Download Congressional Justification 2021 (806 KB PDF)
FY 2021 Budget
DEPARTMENT OF HEALTH AND HUMAN SERVICES
NATIONAL INSTITUTES OF HEALTH
National Institute on Deafness and Other Communication Disorders (NIDCD)
On this page:
- Organization Chart
- Appropriation Language
- Amounts Available for Obligation
- Budget Mechanism Table
- Major Changes in Budget Request
- Summary of Changes
- Budget Graphs
- Budget Authority by Activity
- Authorizing Legislation
- Appropriations History
- Justification of Budget Request
- Budget Authority by Object Class
- Salaries and Expenses
- Detail of Full-Time Equivalent Employment (FTE)
- Detail of Positions
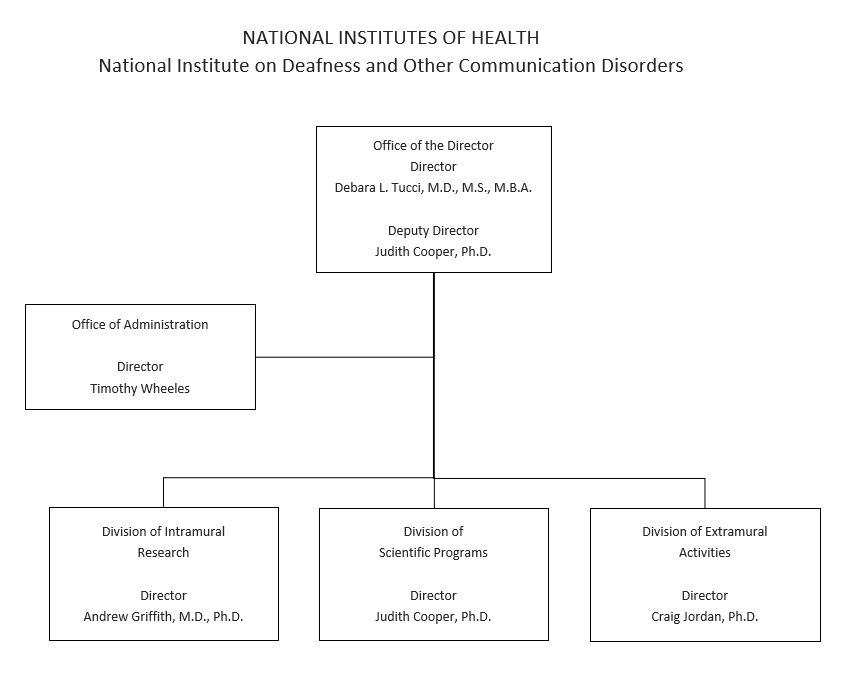
Organization Chart
Appropriation Language
NATIONAL INSTITUTE ON DEAFNESS AND OTHER COMMUNICATION DISORDERS
For carrying out section 301 and title IV of the PHS Act with respect to deafness and other communication disorders, [$490,692,000]$446,397,000.
| (Dollars in Thousands) | |||
| Source of Funding | FY 2019 Final |
FY 2020 Enacted |
FY 2021 President's Budget |
|---|---|---|---|
| 1 Excludes the following amounts (in thousands) for reimbursable activities carried out by this account: FY 2019 - $1,630; FY 2020 - $1,000; FY 2021 - $1,000 |
|||
| Appropriation | $474,404 | $490,692 | $446,397 |
| Mandatory Appropriation: (non-add) | |||
| Type 1 Diabetes | (0) | (0) | (0) |
| Other Mandatory financing | (0) | (0) | (0) |
| Rescission | 0 | 0 | 0 |
| Sequestration | 0 | 0 | 0 |
| Secretary's Transfer | -1,630 | 0 | 0 |
| Subtotal, adjusted appropriation | $472,774 | $490,692 | $446,397 |
| OAR HIV/AIDS Transfers | 222 | 0 | 0 |
| HEAL Transfer from NINDS | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | $472,996 | $490,692 | $446,397 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | $472,996 | $490,692 | $446,397 |
| Unobligated balance lapsing | -8 | 0 | 0 |
| Total obligations | $472,988 | $490,692 | $446,397 |
| (Dollars in Thousands) | ||||||||
| MECHANISM | FY 2019 Final |
FY 2020 Enacted |
FY 2021 President's Budget |
FY 2021 +/- FY2020 Enacted |
||||
|---|---|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | No. | Amount | |
| Research Projects: | ||||||||
| Noncompeting | 614 | $249,191 | 622 | $268,800 | 589 | $248,640 | -33 | -$20,160 |
| Administrative Supplements | (23) | 1,498 | (28) | 2,000 | (14) | 1,000 | (-14) | -1,000 |
| Competing | ||||||||
| Renewal | 34 | 17,382 | 36 | 18,546 | 33 | 17,029 | -3 | -1,517 |
| New | 161 | 64,711 | 142 | 55,637 | 134 | 51,264 | -8 | -4,373 |
| Supplements | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subtotal, Competing | 195 | $82,093 | 178 | $74,183 | 167 | $68,293 | -11 | -$5,890 |
| Subtotal, RPGs | 809 | $332,782 | 800 | $344,983 | 756 | $317,933 | -44 | -$27,050 |
| SBIR/STTR | 38 | 14,639 | 39 | 15,143 | 34 | 13,645 | -5 | -1,498 |
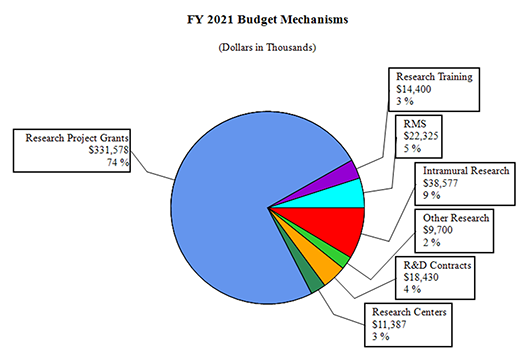
| Research Project Grants | 847 | $347,422 | 839 | $360,126 | 790 | $331,578 | -49 | -$28,548 |
| Research Centers: | ||||||||
| Specialized/Comprehensive | 6 | $14,352 | 7 | $17,016 | 5 | $11,387 | -2 | -$5,629 |
| Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative Medicine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers | 6 | $14,352 | 7 | $17,016 | 5 | $11,387 | -2 | -$5,629 |
| Other Research: | ||||||||
| Research Careers | 46 | $7,698 | 45 | $7,660 | 41 | $7,100 | -4 | -$560 |
| Cancer Education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 28 | 3,050 | 28 | 3,075 | 25 | 2,600 | -3 | -475 |
| Other Research | 74 | $10,748 | 73 | $10,735 | 66 | $9,700 | -7 | -$1,035 |
| Total Research Grants | 927 | $372,521 | 919 | $387,877 | 861 | $352,665 | -58 | -$35,212 |
| Ruth L Kirchstein Training Awards: | FTTPs | FTTPs | FTTPs | FTTPs | ||||
| Individual Awards | 123 | $5,860 | 140 | $6,975 | 130 | $6,400 | -10 | -$575 |
| Institutional Awards | 157 | 8,111 | 155 | 8,040 | 154 | 8,000 | -1 | -40 |
| Total Research Training | 280 | $13,971 | 295 | $15,015 | 284 | $14,400 | -11 | -$615 |
| Research & Develop. Contracts | 41 | $20,686 | 41 | $21,800 | 37 | $18,430 | -4 | -$3,370 |
| (SBIR/STTR) (non-add) | (0) | (157) | (0) | (314) | (0) | (281) | (0) | (-33) |
| Intramural Research | 59 | 42,558 | 65 | 42,500 | 65 | 38,577 | 0 | -3,923 |
| Res. Management & Support | 70 | 23,259 | 75 | 23,500 | 75 | 22,325 | 0 | -1,175 |
| Res. Management & Support (SBIR Admin) (non-add) | (0) | (0) | (0) | (0) | (0) | (0) | (0) | (0) |
| Construction | 0 | 0 | 0 | 0 | ||||
| Buildings and Facilities | 0 | 0 | 0 | 0 | ||||
| Total, NIDCD | 129 | $472,996 | 140 | $490,692 | 140 | $446,397 | 0 | -$44,295 |
| 1All items in italics and brackets are non-add entries. | ||||||||
Major Changes in the Fiscal Year 2021 President’s Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2021 President’s Budget for NIDCD, which is $44.3 million below FY 2020, for a total of $446.4 million.
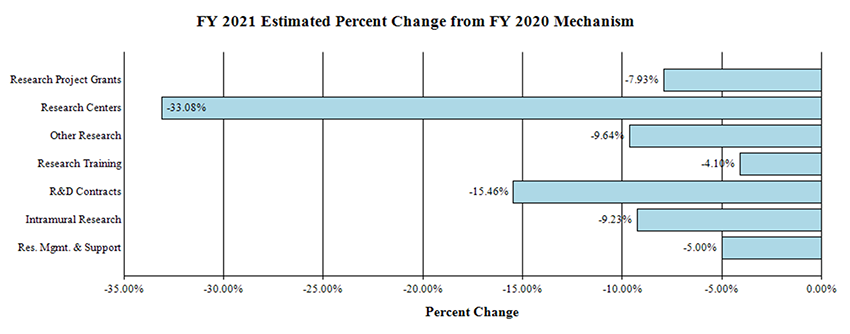
Research Project Grants (RPGs) (-$28.5 million; total $331.6 million):
NIDCD will fund 790 RPG awards in FY 2021, a decrease of 49 awards from the FY 2020 Enacted level. This includes 589 non-competing awards (a decrease of 33 awards and $20.2 million from the FY 2020 Enacted level); 167 competing RPGs (a decrease of 11 awards and $5.9 million from the FY 2020 Enacted level); and 34 SBIR/STTR awards (a decrease of 5 awards and $1.5 million from the FY 2020 Enacted level).
Research Centers (-$5.6 million; total $11.4 million):
NIDCD will fund 5 Center awards in FY 2020, a decrease of 2 from the FY 2020 Enacted level.
Other Research (-$1.0 million; total of $9.7 million):
NIDCD will fund 66 Other Research awards in FY 2021, a decrease of 7 from the FY 2020 Enacted level.
Research Training Awards (-$0.6 million; total $14.4 million):
NIDCD will fund 284 Full-Time Training Positions (FTTPs) in FY 2021, a decrease of 11 from the FY 2020 Enacted level.
| (Dollars in Thousands) | ||||||
| FY 2020 Enacted | $490,692 | |||||
| FY 2021 President's Budget | $446,397 | |||||
| Net change | -$44,295 | |||||
| CHANGES | FY 2021 President's Budget |
Change from FY 2020 Enacted |
||||
|---|---|---|---|---|---|---|
| FTEs | Budget Authority | FTEs | Budget Authority | |||
| A. Built-in: | ||||||
| 1. Intramural Research: | ||||||
| a. Annualization of January 2020 pay increase & benefits | $12,424 | $80 | ||||
| b. January FY 2021 pay increase & benefits | 12,424 | 189 | ||||
| c. Paid days adjustment | 12,424 | -46 | ||||
| d. Differences attributable to change in FTE | 12,424 | 0 | ||||
| e. Payment for centrally furnished services | 6,718 | -354 | ||||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | 19,435 | 39 | ||||
| Subtotal | -$93 | |||||
| 2. Research Management and Support: | ||||||
| a. Annualization of January 2020 pay increase & benefits | $13,105 | $83 | ||||
| b. January FY 2021 pay increase & benefits | 13,105 | 202 | ||||
| c. Paid days adjustment | 13,105 | -49 | ||||
| d. Differences attributable to change in FTE | 13,105 | 0 | ||||
| e. Payment for centrally furnished services | 2,561 | -135 | ||||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | 6,659 | -149 | ||||
| Subtotal | -$48 | |||||
| Subtotal, Built-in | -$140 | |||||
| CHANGES | FY 2021 President's Budget |
Change from FY 2020 Enacted |
||
|---|---|---|---|---|
| No. | Amount | No. | Amount | |
| B. Program: | ||||
| 1. Research Project Grants: | ||||
| a. Nonecompeting | 589 | $249,640 | -33 | -$21,160 |
| b. Competing | 167 | 68,293 | -11 | -5,890 |
| c. SBIR/STTR | 34 | 13,645 | -5 | -1,498 |
| Subtotal, RPGs | 790 | $331,578 | -49 | -$28,548 |
| 2. Research Centers | 5 | $11,387 | -2 | -$5,629 |
| 3. Other Research | 66 | 9,700 | -7 | -1,035 |
| 4. Research Training | 284 | 14,400 | -11 | -615 |
| 5. Research and development contracts | 37 | 18,430 | -4 | -3,370 |
| Subtotal, Extramural | $385,495 | -$39,197 | ||
| FTEs | FTEs | |||
| 6. Intramural Research | 65 | $38,577 | 0 | -$3,830 |
| 7. Research Management and Support | 75 | 22,325 | 0 | -1,127 |
| 8. Construction | 0 | 0 | ||
| 9. Buildings and Facilities | 0 | 0 | ||
| Subtotal, Program | 140 | $446,397 | 0 | -$44,155 |
| Total changes | -$44,295 | |||
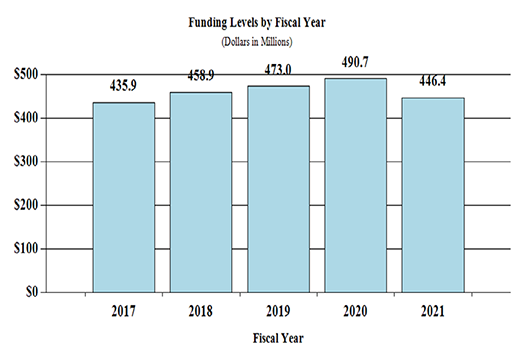
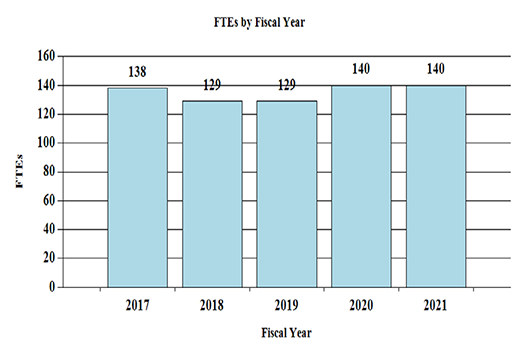
Fiscal Year 2021 Budget Graphs
History of Budget Authority and FTEs:
Distribution by Mechanism:
Change by Selected Mechanisms:
| FY 2019 Final | FY 2020 Enacted | FY 2021 President's Budget | FY 2021 +/- FY2020 | |||||
|---|---|---|---|---|---|---|---|---|
| Extramural Research | FTE | Amount | FTE | Amount | FTE | Amount | FTE | Amount |
| Detail | ||||||||
| Hearing and Balance | $223,670 | $233,291 | $211,759 | -$21,532 | ||||
| Taste and Smell | 62,557 | 65,247 | 59,225 | -6,022 | ||||
| Voice, Speech, and Language | 120,952 | 126,154 | 114,511 | -11,643 | ||||
| Subtotal, Extramural | $407,179 | $424,692 | $385,495 | -$39,197 | ||||
| Intramural Research | 59 | $42,558 | 65 | $42,500 | 65 | $38,577 | 0 | -$3,923 |
| Research Management & Support | 70 | $23,259 | 75 | $23,500 | 75 | $22,325 | 0 | -$1,175 |
| TOTAL | 129 | $472,996 | 140 | $490,692 | 140 | $446,397 | 0 | -$44,295 |
| 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | ||||||||
| PHS Act/Other Citation | U.S. Code Citation | 2020 Amount Authorized | FY 2020 Enacted | 2021 Amount Authorized | FY 2021 President's Budget | |
|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinite |  $490,692,000 $490,692,000 |
Indefinite |  $446,397,000 $446,397,000 |
| National Institute on Deafness and Other Communication Disorders | Section 401(a) | 42§281 | Indefinite | Indefinite | ||
| Total, Budget Authority | $490,692,000 | $446,397,000 |
| Fiscal Year | Budget Estimate to Congress | House Allowance |
Senate Allowance |
Appropriation |
|---|---|---|---|---|
|
1 Budget Estimate to Congress includes mandatory financing. |
||||
| 2012 | $426,043,000 | $426,043,000 | $410,482,000 | $417,061,000 |
| Rescission | $788,245 | |||
| 2013 | $417,297,000 | $418,562,000 | $416,272,755 | |
| Rescission | $832,546 | |||
| Sequestration | ($20,894,030) | |||
| 2014 | $422,936,000 | $420,125,000 | $404,049,000 | |
| Rescission | $0 | |||
| 2015 | $403,933,000 | $405,302,000 | ||
| Rescission | $0 | |||
| 2016 | $416,241,000 | $412,366,000 | $424,860,000 | $423,031,000 |
| Rescission | $0 | |||
| 20171 | $422,936,000 | $434,126,000 | $441,778,000 | $436,875,000 |
| Rescission | $0 | |||
| 2018 | $325,846,000 | $443,624,000 | $451,768,000 | $459,974,000 |
| Rescission | $0 | |||
| 2019 | $423,992,000 | $465,467,000 | $474,653,000 | $474,404,000 |
| Rescission | $0 | |||
| 2020 | $408,358,000 | $497,590,000 | $500,270,000 | $490,692,000 |
| Rescission | $0 | |||
| 2021 | $446,397,000 | |||
| FY 2019 Final | FY 2020 Enacted | FY 2021 President's Budget | FY 2021 +/- FY 2020 |
||||
|---|---|---|---|---|---|---|---|
| BA | $472,996,000 | $490,692,000 | $446,397,000 | -$44,295,000 | |||
| FTE | 129 | 140 | 140 | 0 | |||
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director's Overview
At least 46 million people in the United States have a hearing or other communication disorder.1 For these individuals, the basic components of communication – sensing, interpreting, and responding to people and things in our environment – can be challenging. The National Institute on Deafness and Other Communication Disorders (NIDCD) manages a broad portfolio of basic, translational, clinical, and public health research focused on human communication and associated disorders in three program areas: hearing and balance; taste and smell; and voice, speech, and language.
Highlights of NIDCD Research Advances from Mission Areas:
Extraordinary research opportunities have led to scientific breakthroughs in the study of genes, proteins, cellular and molecular processes, neural circuits, and sensory and motor systems that directly affect our understanding of communication disorders. For example, NIDCD-supported research has led to advances in the following areas of communication science:
New Discoveries Could Help Treat Hereditary Hearing Loss:2 NIDCD has long been interested in the identification of genetic mutations associated with hereditary hearing loss. For example, in 1995, NIDCD scientists identified the first genetic mutation associated with Usher syndrome, a rare disorder that affects hearing, balance, and vision. Individuals with Usher syndrome have profound loss of hearing and balance because the sensory hair cells in the inner ear develop and function abnormally. By 2013, at least 10 additional Usher syndrome genetic mutations had been identified. Researchers recently inserted one Usher syndrome mutation into zebrafish and successfully identified the process by which the genetic mutation disrupted sensory hair cell function. Further, the scientists discovered that a common antimalarial drug, artemisinin, could counteract this disruption and preserve sensory hair cell function in treated zebrafish. NIDCD plans to translate this finding in zebrafish to mammals and eventually to humans with Usher Syndrome. NIDCD will continue to strive to discover new genetic mutations that may cause rare types of hearing loss and balance disorders and to use this information to develop new therapies to treat affected individuals.
Identification of an Elusive Sour Taste Receptor:3,4 Taste plays a critical role in helping us determine what is safe to eat and what might be poisonous. Although we know how most tastes are detected, scientists have been searching for the elusive receptor that detects sour taste. In 2018, NIDCD-supported scientists identified a likely candidate when they discovered how a protein called OTOP1 forms channels that allow protons to enter sour taste receptor cells on the tongue. Recently, NIDCD-supported scientists described the three-dimensional structure and electrostatic charge interactions of residues within channel proteins OTOP1 and 3 to determine how they control cellular proton entry. Now, the same scientists collaborated on another NIDCD-supported study to test sour taste sensitivity in mice that lack the gene (Otop1) needed to make the OTOP1 channel protein. The sour taste cells in mice lacking OTOP1 had reduced responses to sour-tasting liquids, providing evidence that OTOP proteins play a critical role in detecting sour tastes on the tongue. Understanding how we perceive sour taste will provide insight into why we seek out particular foods and avoid others. NIDCD will continue to support research focused on understanding the basic mechanisms of taste and smell to help understand how taste and smell contribute to flavor perception and food selection.
Scientists Discover Brain Cells Involved in Stuttering:5 Stuttering occurs primarily in young children, most of whom eventually outgrow the problem. However, for one quarter of children with early onset stuttering, the condition persists as a life-long communication problem. For several decades, scientists have been unable to identify the causes of persistent stuttering. A breakthrough occurred 10 years ago when NIDCD scientists identified the first genetic mutations in humans associated with stuttering. Now, in a recent advance, NIDCD researchers identified changes in the brain brought on by one of the gene mutations previously linked to stuttering. The scientists made this discovery by engineering one of the human stuttering gene mutations into mice and then studying the mouse vocalizations. They found that mice with the mutation had long pauses in their streams of vocalizations, somewhat similar to those found in the speech of people with the same mutation. The scientists also noticed these mice had a loss of astrocytes – a type of brain supporting cell – in the corpus callosum, which is a part of the brain that enables communication between the brain’s left and right hemispheres. The corpus callosum helps integrate signals for processes that involve both hemispheres, such as physical coordination and use of language. The identification of genetic, molecular, and cellular changes that underlie stuttering has led scientists to understand persistent stuttering as a brain disorder. Future research will focus on understanding what brain regions and specific cells are involved, in order to open opportunities for novel interventions for stuttering – and possibly other speech disorders.
NIDCD Plans, Priorities, and Challenges for the Future: Over the past 30 years, researchers supported by the NIDCD have made seminal advances in understanding the normal and disordered processes of hearing, balance, taste, smell, voice, speech, and language, leading to increasingly effective, evidence-based treatments for conditions that affect an ever growing segment of the U.S. population. Moving forward, NIDCD staff, with the guidance of the Institute’s advisory committees – the National Deafness and Other Communication Disorders Advisory Council and the Board of Scientific Counselors – will examine NIDCD’s current portfolio and priorities, opportunities for partnerships, and the needs of the Institute’s targeted populations and constituencies to develop a strategic plan with a vision toward the future. NIDCD expects current efforts to culminate in a new research strategic plan in calendar year 2022. The Institute’s vision is that all Americans benefit from scientific discovery that informs effective and accessible treatments and improves quality of life.
NIDCD continues its support of new and competing Research Project Grants from investigators who have innovative ideas. NIDCD prioritizes its research investments to identify the most promising opportunities for research on deafness and other communication disorders and is committed to identifying effective interventions for the treatment or prevention of communication disorders by supporting well-designed and well-executed clinical trials. NIDCD funding announcements reflect investments in clinical trials that advance the care of individuals with deafness and other communication disorders.6,7 Further, NIDCD supports an announcement on “Translating Basic Research into Clinical Tools”8 which provides an avenue for basic research scientists, clinicians, and clinical scientists to translate basic research into practical applications.
NIDCD prioritizes and supports Early-Stage Investigators (ESIs), defined as investigators who are within 10 years of receiving their terminal educational degree. The Institute has a long history of supporting ESIs, through early stages of career development with special programs including the Early Career Research Award (implemented in 1990) and the expedited review of pre- and post-doctoral Fellowship applications (implemented in 2000). In 2019, five NIDCD ESIs were honored with the Presidential Early Career Award for Scientists and Engineers, the highest honor bestowed by the Federal Government on outstanding scientists beginning their independent career. NIDCD’s longstanding support of diverse, interdisciplinary ESIs ensures a robust pipeline of scientists pursuing research to tackle the world’s complex public health needs.
Overall Budget Policy:
The FY 2021 President’s Budget request for NIDCD is $446.4 million, a decrease of $44.3 million or 9.0 percent compared with the FY 2020 Enacted level.
Program Descriptions and Accomplishments
Hearing and Balance Program: Loss of hearing or balance imposes a significant social and economic burden upon individuals, their families, and the communities in which they live. Hearing and balance disorders cross all ethnic and socioeconomic lines. Among adults, hearing loss is the third most common chronic condition in the United States.9 The prevalence of hearing loss increases with age, with nearly 25 percent of adults aged 65-74 having a disabling hearing loss.10 This rate increases to 50 percent for those who are 75 and older.10 This fact, along with the aging demographic in the United States will account for an increasing burden of age-related hearing loss in the years ahead. About 2 out of every 1,000 children in the United States are born with a detectable level of hearing loss in one or both ears that can affect their speech, language, social, and cognitive development.11 More than 1 in 20 American children have a dizziness or balance problem.12 Accordingly, NIDCD supports research that will lead to improved treatments for, and prevention of, hearing and balance disorders. These research projects encompass over half of NIDCD’s grant portfolio.
Ear infections are one of the most common reasons parents bring their child to a doctor. In some of these cases, the ear infection can persist or recur. NIDCD-supported scientists are leading research to discover how a common bacterium, Haemophilus influenzae, causes recurrent ear infections.13 By creating biofilms, a thin film of bacteria that adheres on the surface of the middle ear, the bacteria create a protective blanket that shields them from immune system responses and antibiotic treatments. Using a mouse model, researchers discovered a bacterial scaffolding that is important for creating and maintaining biofilms, thus explaining why some infections may linger or reoccur. By targeting this structure, the researchers prevented biofilms from forming and broke apart existing biofilms, making it easier to permanently kill the bacteria with antibiotics.14 In future work, scientists will study whether treatments that break down biofilms could help prevent persistent ear infections.
Roughly 10 percent of American adults have experienced tinnitus, often described as a ringing in the ears.15 For many, tinnitus can be severe and unremitting, and – especially in these cases – can be associated with decreased quality of life. While there is no cure, some interventions can reduce the severity. NIDCD supports research to determine the neural basis of tinnitus and to identify behavioral, pharmacological, surgical, and device-based treatments for improving tinnitus symptoms.16 For example, NIDCD-supported scientists conducted the first clinical trial comparing the standard of care (patient-centered counseling) to Tinnitus Retraining Therapy (patient-centered counseling and the use of a sound generator to help tune out tinnitus).17 The researchers found no added benefit of the sound generator device, which can be very costly. In another example, researchers are exploring brain stimulation to treat difficult tinnitus cases. Hyperactivity in a region of the brainstem that integrates sound and touch information may underlie tinnitus in some individuals.18 Using this information, researchers piloted a novel and non-invasive treatment for tinnitus by stimulating this brain area in rodents and humans.19 Another group of NIDCD-funded researchers performed a clinical trial of deep brain stimulation in a different brain area – an area which links sound information with emotional state.20 These treatments show promise for those who experience tinnitus. Future clinical trials could allow for the commercialization of these techniques.
A balance disorder is a disabling condition, is common among the elderly, and is one of the primary causes of falls. In 2015, the total medical costs for falls totaled more than $50 billion.21 Medicare and Medicaid bore 75 percent of these costs.21 To understand better how aging affects the vestibular system, NIDCD and the National Institute on Aging (NIA) convened a scientific workshop in 2019 on the Central and Peripheral Control of Balance in Older Adults. The workshop focused on the following topics: balance and the aging brain; diagnosis and assessment of balance disorders; leveraging new technologies in the assessment and management of balance disorders; and balance interventions, therapies, and targets for treatments. Representatives with expertise in basic, clinical, epidemiologic and population health research in the fields of gerontology, geriatrics, vestibular physiology, neurology, neuroscience, movement science, movement disorders, and rehabilitation participated in the workshop. The discussions provided NIDCD and NIA with a research blueprint to increase our understanding of the aging of the vestibular system and facilitate effective management of mobility disability, falls, and cognitive impairment in older adults.
There are currently no effective treatments for some balance disorders, which may be caused by damaged vestibular hair cells in the inner ear. In a landmark study, NIDCD-supported scientists were the first to restore the sense of balance in mice by manipulating a protein that regulates the hair cells in the inner ear that are responsible for balance.22 This discovery may be an important first step towards treating vestibular disorders, or dizziness. In the study, the scientists damaged vestibular hair cells in mice and discovered that approximately one third of the hair cells regenerated spontaneously but appeared immature and the mice did not recover their balance. The scientists then engineered a protein, Atoh1, which regulates inner ear hair cell formation, to be expressed in abundance. In the mice that overexpressed Atoh1, as much as 70 percent of vestibular hair cells regenerated and about 70 percent of these mice recovered their balance. This finding opens the door to new research that may lead to treatment for the 35 million American adults who report having a balance or dizziness problem.
PROGRAM PORTRAIT: Ending the 40-Year Quest to Find the Hearing and Balance Key Sensory Protein
NIDCD-supported scientists have identified the key sensory protein for hearing and balance, TMC1, which is responsible for converting sound and movement into electrical signals that the brain can interpret. The key proteins for most other senses have been identified, but this pivotal hearing protein has been elusive for more than 40 years.
The TMC1 protein, abbreviated from “transmembrane cochlear-expressed gene 1,” forms an ion channel located in the membrane of sensory hair cells in the inner ear. These hair cells are found in the cochlea (the organ responsible for hearing) and in the semi-circular canals (the structures responsible for balance and motion detection). Sound vibrations and head movements cause stereocilia (bristly projections that perch atop the hair cells) to bend, thus causing the TMC1 channel to open letting positively charged ions into the cell. This process, called mechanotransduction, converts a mechanical signal into an electrical signal that the brain can interpret as sound or movement. Mechanotransduction from the inner ear to the brain was first discovered in the 1970s, but no one knew which genes and their corresponding proteins were responsible.
Researchers identified TMC1 in 2002 by conducting gene mapping studies of a large North American family with deafness and 11 unrelated families from Pakistan and India with the same kind of hereditary deafness.23 The researchers found that in all 12 families, 1 of 8 different mutations in the TMC1 gene was responsible for the hearing loss. Animal models with mutations in the mouse version of TMC1 were also profoundly deaf, and tests showed that the cochlear hair cells showed no electrical response to sound stimulation. While the newly discovered gene was important for normal hearing, scientists were not able to determine if it was the gatekeeper for mechanotransduction.
Almost 10 years later, NIDCD-supported scientists discovered another piece of the puzzle.24 Using genetically engineered mice where the TMC1 gene was deleted, the researchers found a specific functional deficit in the mechanotransduction channels of the mice’s stereocilia, but the rest of the hair cell’s structure and function was normal. When the researchers used gene therapy to re-introduce a functional copy of TMC1 to hair cells, they saw that mechanotransduction was restored.
In 2013, NIDCD-supported scientists found that changing one base or “letter” in the genetic sequence of TMC1 was enough to change, but not destroy, the properties and function of the ion channels that resulted in progressive deafness.25 A later study used powerful microscopy techniques to pinpoint the precise cellular location of the TMC1 protein in the inner ear of mice.26 They found that TMC1 was localized predominately in the stereocilia tips, the site of mechanotransduction, providing further evidence that TMC1 was likely part of the elusive hair cell mechanotransduction channel.
In 2018, a team of NIDCD-supported scientists studied the precise structure and function of the amino acids that made up the TMC1 protein.27 They identified the region of the protein that was hypothesized to make up the channel (or pore). They substituted the 17 amino acids of the pore region one at a time to gauge whether and how each substitution altered the hair cells’ ability to respond to sound and affected the flow of ions. Of the 17 substitutions, 11 altered the influx of ions, and 5 did so dramatically, reducing flow by up to 80 percent. One substitution blocked ion influx, a finding that confirmed the precise location of the pore that initiates mechanotransduction. This finding helped definitively confirm that TMC1 was a component of the mechanotransduction channel for hearing and balance.
Knowing the precise structure and function of TMC1 enables scientists to gain new insights into disorders of hearing and balance. This discovery also lays the groundwork for precision-targeted therapies to treat hearing loss when the TMC1 gene is mutated or missing. NIDCD will continue to fund basic research to define the spectrum of genetic contributions to inherited hearing loss and to understand the structural and functional consequences of such mutations with the goal to develop new therapies to prevent hearing loss or restore hearing and balance function.
Budget Policy:
The FY 2021 President’s Budget request for the Hearing and Balance program is $211.8 million, a decrease of $21.5 million or 9.2 percent compared with the FY 2020 Enacted level. In FY 2021, the program will continue emphasizing faculty, postdoctoral, and student training on hearing and balance sciences. Postdoctoral fellows who show high promise as emerging independent investigators will be encouraged to launch their careers through the Pathways to Independence (K99-R00) program. Making R01 awards to first-time and early-stage investigators in the area of hearing and balance sciences will be given funding priority.
Taste and Smell Program: Taste and smell disorders are underreported, and often undiagnosed. NIDCD supports studies of the chemical senses known as taste, smell, and chemesthesis (chemically provoked irritation) to enhance our understanding of how individuals gather information about their environment and how human chemosensory disorders can be diagnosed and treated. With age, the olfactory (sense of smell) system is less able to repair and regenerate, and our sense of smell declines. Although prevalence estimates vary, it is likely that more than one third of adults over the age of 70 have olfactory deficits.28 Since both taste and smell contribute to flavor perception, such olfactory deficits affect the flavor of foods and consequently negatively affect food intake, diet, overall nutrition, and health status. NIDCD encourages further studies of age-related decline in olfactory sensitivity including the development of better diagnostic tests to assess chemosensory loss and animal models to study why this decline occurs and how to prevent or treat it.
NIDCD supports basic research to understand how mosquitos “smell” humans in order to control and prevent insect-borne diseases. The mosquito Aedes aegypti, which transmits dengue, Zika, yellow fever, and chikungunya, is adapted to life in populated areas. Female Ae. aegypti mosquitos preferentially seek out humans for blood meals to develop their eggs. Scientists are hoping to learn how to prevent mosquitos from biting and spreading disease by understanding the signaling molecules that drive mosquito feeding behavior. After a blood meal, female mosquitos temporarily lose interest in seeking more blood. NIDCD-supported scientists have combined powerful molecular tools with studies of mosquito behavior to identify a receptor – Neuropeptide Y-like receptor 7 (NPYLR7) – that, when activated, prevents mosquitos from seeking human blood meals.29 Scientists hope to develop ways to deliver molecules that activate NPYLR7 to mosquitos to prevent human blood-seeking and prevent subsequent mosquito-borne illnesses.
Our sense of taste plays a broader role than determining what we eat – in some cases, it can also determine whether we take lifesaving medicines. The bitter taste of HIV treatment drugs such as KaletraTM, for example, causes some children to refuse to take this important medication. Taste scientists know that people demonstrate a range of variations in preference and avoidance of different tastes. Although scientists suspect that variations in genes underly individual taste preference, we still don’t know which genes underly which preferences. NIDCD-supported scientists tested “palatability,” or taste acceptability, of the HIV medicine KaletraTM on a panel of adult tasters, and looked at gene expression – or genotype – in the individuals who accepted the taste as compared to those who could not tolerate it.30 They discovered that a panelist’s palatability of KaletraTM varied along with expression of two particular taste-related genes, and that panelists gave the same palatability ratings on subsequent tests. The next step will be to determine whether the same patterns of taste preference variation observed in adults are also true for children. Scientists may then be ready to develop tools to predict which children will reject
the taste of certain drugs.
Budget Policy:
The FY 2021 President’s Budget request for the Taste and Smell program is $59.2 million, a decrease of $6.0 million or 9.2 percent compared with the FY 2020 Enacted level. In FY 2020, the program will continue emphasizing faculty, postdoctoral, and student training on smell and taste sciences. Postdoctoral fellows who show high promise as emerging independent investigators will be encouraged to launch their careers through the Pathways to Independence (K99-R00) program. Making R01 awards to first-time and early-stage investigators in the area of smell and taste sciences will be given funding priority.
Voice, Speech, and Language Program: Disorders involving voice, speech, or language can have an overwhelming effect on an individual’s health and quality of life. These disorders affect people of all ages with or without hearing impairment, such as children with autism, people who stutter, and adults with acquired communication disorders. Voice, speech, and language disorders also come at a significant cost. The societal burden of laryngeal disorders is estimated at $11 billion annually due to work-related disability, lost productivity, and direct health care cost.31 Further, nearly eight percent of children ages 3-17 years have had a communication disorder during the past 12 months, according to data from the National Health Interview Survey, 2012.32 By the first grade, roughly five percent of children have noticeable speech disorders.33 In children, delayed speech and language acquisition or impairment are very often significant predictors of future academic, social, vocational, and adaptive outcomes.34
Autism spectrum disorders (ASD) are a group of developmental disabilities that can cause significant social, communication, and behavioral challenges. According to the Centers for Disease Control and Prevention, about 1 in 59 children is diagnosed with ASD. NIDCD has identified a number of research needs and opportunities related to children with autism, and in particular, among the 25-30 percent of children with ASD who remain functionally non-verbal beyond 5 years of age – a subset under-represented in autism research. Many infants at risk for ASD show differences in the development of social attention and early forms of communication over the first year of life, but reliable assessment measures for these developmental delays are lacking. NIDCD supports research to examine and measure differences in speech and language abilities in ASD individuals. In one study, NIDCD-supported scientists are using functional imaging to discover neural biomarkers for language and social development in ASD toddlers. These researchers found distinct neurobiology and gene expression in ASD subtypes with variable early language outcomes – well before such outcomes were known.35 In another study funded in part by NIDCD, scientists were able to accurately predict ASD in babies as young as three months old based on their brain’s electrical activity.36 Further, NIDCD is participating in two funding opportunity announcements to support research for “Early Screening for ASD,”37,38 to improve the early diagnosis of ASD and ultimately to develop effective treatments to address the communication challenges faced by many with ASD. As a result, NIDCD co-funded one project with relevance to our mission area.
NIDCD also supports basic, clinical, and translational research on voice disorders for individuals that have impaired function due to injury or disease. Vocal fold paralysis (also known as vocal cord paralysis) is a voice disorder that occurs when one or both of the vocal folds don't open or close properly. Single vocal fold paralysis is a common disorder. Paralysis of both (bilateral) vocal folds (BVFP) is rare and can be life threatening because it causes serious problems with breathing. Treatment involves cutting the vocal fold to improve ventilation, but the surgery damages the voice and compromises swallowing. NIDCD supports a clinical trial to develop an electrical stimulation technology to help people with BVFP avoid surgery and improve vocal fold function.39 The device, which currently is being tested in animals and people, uses an implanted pacemaker to stimulate laryngeal nerves. Researchers hope that bilateral stimulation will return mobility to the vocal folds so that they can open to allow breathing and close to allow speaking and swallowing.
Augmentative and alternative communication (AAC) devices help people with voice, speech, or language disorders communicate. People with amyotrophic lateral sclerosis (or motor neuron disease) or brainstem stroke lose their ability to move their arms, legs, or body. They can also become locked-in, where they are not able to speak, even though they are able to think and reason normally. However, with the advent of brain-computer interface (BCI) technology, which uses sensors to measure electrical signals in the brain to run a computer, NIDCD scientists believe they have found a way to restore hope to these individuals. In an important advance, NIDCD-supported engineers developed an approach to turn a person’s thoughts directly into speech.40 The scientists trained a computer algorithm – that is typically used to interpret voice commands – to decipher brain activity instead. Individuals with epilepsy undergoing brain surgery listened to sentences spoken by different people. The researchers used the neural patterns measured from these patients to train the computer algorithm. When the patients simply thought of the words they had heard, the algorithm was able to accurately decipher the thoughts 75 percent of the time. NIDCD will continue to invest in assistive communication devices, such as BCI technology, to help people with amyotrophic lateral sclerosis, stroke, or neurodegenerative disease regain their ability to communicate.
Budget Policy:
The FY 2021 President’s Budget request for the Voice, Speech, and Language program is $114.5 million, a decrease of $11.6 million or 9.2 percent compared with the FY 2020 Enacted level. In FY 2021, the program will continue emphasizing faculty, postdoctoral, and student training on voice, speech, and language sciences. Postdoctoral fellows who show high promise as emerging independent investigators will be encouraged to launch their careers through the Pathways to Independence (K99-R00) program. Making R01 awards to first-time and early-stage investigators in the area of voice, speech, and language sciences will be given funding priority.
Intramural Research Program (IRP): The NIDCD Intramural Research Program conducts basic and clinical research in human communication, with a primary focus on hearing and balance.
Other NIDCD intramural scientists are taking advantage of the Cancer Genome Atlas (TCGA) consortium, a joint effort of the NIH’s National Cancer Institute and National Human Genome Research Institute that is a culmination of more than a decade of work by more than 150 researchers across North America. They studied the genomic profiles of a type of head and neck cancer, called squamous cell carcinoma (SSC), to develop targeted treatments. They uncovered molecular characteristics that distinguish the genomic profiles of SCCs apart from other cancers.41 This research can lead to more effective diagnosis and treatment of these cancers by helping researchers develop tailored strategies for specific cancer subtypes. Here, the researchers went on to explore the impact of a specific micro-RNA that was reduced in these cancers. They found that this small molecule helps regulate signaling networks and suppress tumors.42 Since the loss of this micro RNA likely reduces people’s ability to fight off the cancer, therapies to increase its levels in the body could be a precision-medicine approach to treating these types of cancer.
In another intramural project, scientists are exploring the cellular implications of variations of a gene, TRIO and F-Actin Binding Protein (TRIOBP), which has been known to cause profound deafness in humans and mice.43 Using mice as a model, the researchers learned that this gene is vital to development of key sensory structures in the inner ear called stereocilia. Stereocilia sit atop hair cells in the inner ear and sway with vibrations caused by sound. This motion causes the hair cell to send a signal to the brain which is interpreted as sounds we recognize and understand. The researchers discovered that TRIOBP is involved in supporting the cellular skeleton of the stereocilia. A protein called actin makes the core of this skeleton inside the stereocilia and forms a root to connect it securely to the hair cell. The researchers found that the variations of the TRIOBP gene weaken the actin root, causing the stereocilia to be floppy and unable to respond appropriately to sound vibrations. This research helps explain how we hear, why mutations in TRIOBP cause deafness, and hints at possibilities for future therapies for this gene variation.
Menthol provides a minty taste and a cooling or soothing sensation but long has played a particularly troubling role in U.S. cigarette smoking patterns. Nearly 20 million people in the United States smoke menthol cigarettes, which are particularly popular among African-American and teen smokers.44 NIDCD intramural scientists identified a genetic variant found only in a subset of people of African descent that significantly increases a smoker’s preference for cigarettes containing menthol.45 The gene variant is five to eight times more frequent among smokers who use menthol cigarettes than other smokers. The multiethnic study is the first to look across all genes to identify genetic vulnerability to menthol cigarettes. This finding could inform policies on menthol flavoring in combustible and e-cigarettes.
Budget Policy:
The FY 2021 President’s Budget request for the Intramural Research program is $38.6 million, a decrease of $3.9 million or 9.2 percent compared with the FY 2020 Enacted level. The NIDCD Intramural Research Program has been a leader in research on hereditary hearing impairment and has identified many genes whose mutation causes hearing loss. In FY 2021, NIDCD intramural scientists will continue their efforts in identifying mutation in genes, which impacts hearing, and identifying or studying additional genes involved in communication disorders in humans and animal models.
Research Management and Support (RMS) Program: NIDCD RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS functions also include strategic planning, coordination, and evaluation of the Institute’s programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. The Institute currently supports approximately 1,400 research grants, training awards, and research and development (R&D) contracts.
Dr. Debara L. Tucci became the NIDCD Director on September 3, 2019. She is the NIDCD’s first woman in this role and the institute’s fourth director since it was founded in 1988. Dr. Tucci has been recognized as a pioneer in her work to understand the causes and impact of hearing loss and to develop treatments to restore hearing. Prior to joining NIDCD, she was on the faculty of Duke University Medical Center in Durham, North Carolina, for more than 25 years, where she co-founded the Duke Hearing Center and directed the medical center’s cochlear implant program. Dr. Tucci’s vision is that all people with disorders of hearing, balance, taste, smell, voice, speech, or language will benefit from scientific discovery that informs effective and accessible treatments and improves quality of life.
Budget Policy:
The FY 2021 President’s Budget request for the Research Management and Support program is $22.3 million, a decrease of $1.2 million or 5.0 percent compared with the FY 2020 Enacted level.
| FY 2020 Enacted | FY 2021 President's Budget | FY 2021 +/- FY 2020 |
|
|---|---|---|---|
| 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||
| Total compensable work years | |||
| Full-time equivalent | 140 | 140 | 0 |
| Full-time equivalent of overtime and holiday hours | 0 | 0 | 0 |
| Average ES salary | $192 | $192 | $0 |
| Average GM/GS grade | 12.4 | 12.4 | 0.0 |
| Average GM/GS salary | $122 | $123 | $1 |
| Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) |
$134 | $138 | $4 |
| Average salary of ungraded positions | $107 | $108 | $1 |
| OBJECT CLASSES | FY 2020 Enacted | FY 2021 President's Budget | FY 2021 +/- FY2020 |
| Personnel Compensation | |||
| 11.1 Full-Time Permanent | 11,984 | 12,122 | 138 |
| 11.3 Other Than Full-Time Permanent | 4,815 | 4,870 | 55 |
| 11.5 Other Personnel Compensation | 411 | 416 | 5 |
| 11.7 Military Personnel | 130 | 134 | 3 |
| 11.8 Special Personnel Services Payment | 1,572 | 1,590 | 18 |
| 11.9 Subtotal Compensation | $18,913 | $19,132 | $219 |
| 12.1 Civilian Personnel Benefits | 6,096 | 6,333 | 238 |
| 12.2 Military Personnel Benefits | 61 | 63 | 2 |
| 13.0 Benefits to Former Personnel | 0 | 0 | 0 |
| Subtotal Pay Costs | $25,070 | $25,529 | $459 |
| 21.0 Travel & Transportation of Persons | 338 | 141 | -197 |
| 22.0 Transportation of Things | 26 | 6 | -19 |
| 23.1 Rental Payments to GSA | 0 | 0 | 0 |
| 23.2 Rental Payments to others | 0 | 0 | 0 |
| 23.3 Communication, Utilities & Misc. Charges | 117 | 119 | 2 |
| 24.0 Printing & Reproduction | 0 | 0 | 0 |
| 25.1 Consulting Services | 135 | 62 | -72 |
| 25.2 Other Services | 7,618 | 5,474 | -2,144 |
| 25.3 Purchase of goods and services from government account | 46,420 | 42,291 | -4,129 |
| 25.4 Operation & Maintenance of Facilities | 165 | 165 | 0 |
| 25.5 R&D Contracts | 3,073 | 2,548 | -525 |
| 25.6 Medical Care | 204 | 212 | 8 |
| 25.7 Operation & Maintenance of Equipment | 1,031 | 1,052 | 21 |
| 25.8 Subsistence & Support of Persons | 24 | 25 | 0 |
| 25.0 Subtotal Other Contractual Services | $58,670 | $51,828 | -$6,841 |
| 26.0 Supplies & Materials | 1,890 | 928 | -962 |
| 31.0 Equipment | 1,690 | 781 | -909 |
| 32.0 Land and Structures | 0 | 0 | 0 |
| 33.0 Investment & Loans | 0 | 0 | 0 |
| 41.0 Grants, Subsidies & Contributions | 402,892 | 367,065 | -35,827 |
| 42.0 Insurance Claims & Indemnities | 0 | 0 | 0 |
| 43.0 Interest & Dividends | 0 | 0 | 0 |
| 44.0 Refunds | 0 | 0 | 0 |
| Subtotal Non-Pay Costs | $465,622 | $420,868 | -$44,754 |
| Total Budget Authority by Object Class | $490,692 | $446,397 | -$44,295 |
| OBJECT CLASSES | FY 2020 Enacted | FY 2021 President's Budget | FY 2021 +/- FY2020 |
|---|---|---|---|
| Personnel Compensation | |||
| Full-Time Permanent (11.1) | $11,984 | $12,122 | $138 |
| Other Than Full-Time Permanent (11.3) | 4,815 | 4,870 | 55 |
| Other Personnel Compensation (11.5) | 411 | 416 | 5 |
| Military Personnel (11.7) | 130 | 134 | 3 |
| Special Personnel Services Payments (11.8) | 1,572 | 1,590 | 18 |
| Subtotal Personnel Compensation (11.9) | $18,913 | $19,132 | $219 |
| Civilian Personnel Benefits (12.1) | $6,096 | $6,333 | $238 |
| Military Civilian Personnel Benefits (12.2) | 61 | 63 | 2 |
| Benefits to Former Personnel (13.0) | 0 | 0 | 0 |
| Subtotal Pay Costs | $25,070 | $25,529 | $459 |
| Travel & Transportation of Persons (21.0) | $338 | $141 | -$197 |
| Transportation of Things (22.0) | 26 | 6 | -19 |
| Rental Payments to Others (23.2) | 0 | 0 | 0 |
| Communications, Utilities & Misc. Charges (23.3) | 117 | 119 | 2 |
| Printing & Reproduction (24.0) | 0 | 0 | 0 |
| Other Contractual Services | |||
| Consultant Services (25.1) | 135 | 62 | -72 |
| Other Services (25.2) | 7,618 | 5,474 | -2,144 |
| Purchases from government accounts (25.3) | 30,434 | 28,878 | -1,557 |
| Operation & Maintenance of Facilities (25.4) | 165 | 165 | 0 |
| Operation & Maintenance of Equipment (25.7) | 1,031 | 1,052 | 21 |
| Subsistence & Support of Persons (25.8) | 24 | 25 | 0 |
| Subtotal Other Contractual Services | $39,407 | $35,656 | -$3,751 |
| Supplies and Materials (26.0) | $1,890 | $928 | -$962 |
| Subtotal Non-Pay Costs | $41,777 | $36,849 | -$4,928 |
| Total Administrative Costs | $66,847 | $62,378 | -$4,469 |
| OFFICE/DIVISION | FY 2019 Final | FY 2020 Enacted | FY 2021 President's Budget | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Division of Extramural Activities | |||||||||
| Direct: | 19 | - | 19 | 20 | - | 20 | 20 | - | 20 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 19 | - | 19 | 20 | - | 20 | 20 | - | 20 |
| Division of Intramural Research Program | |||||||||
| Direct: | 56 | 1 | 57 | 64 | 1 | 65 | 64 | 1 | 65 |
| Reimbursable: | 2 | - | 2 | - | - | - | - | - | - |
| Total: | 58 | 1 | 59 | 64 | 1 | 65 | 64 | 1 | 65 |
| Division of Scientific Programs | |||||||||
| Direct: | 16 | - | 16 | 18 | - | 18 | 18 | - | 18 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 16 | - | 16 | 18 | - | 18 | 18 | - | 18 |
| Office of Administration | |||||||||
| Direct: | 33 | - | 33 | 35 | - | 35 | 35 | - | 35 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 33 | - | 33 | 35 | - | 35 | 35 | - | 35 |
| Office of the Director | |||||||||
| Direct: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Total: | 128 | 1 | 129 | 139 | 1 | 140 | 139 | 1 | 140 |
| Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||||||||
| FTEs supported by funds from Cooperative Research and Development Agreements. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
|
|||||||||
| Fiscal Year | Average GS Grade | ||||||||
| 2017 | 12.1 | ||||||||
| 2018 | 12.4 | ||||||||
| 2019 | 12.4 | ||||||||
| 2020 | 12.4 | ||||||||
| 2021 | 12.4 | ||||||||
| GRADE | FY 2019 Final | FY 2020 Enacted | FY 2021 President's Budget |
|---|---|---|---|
| 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | 192,254 | 192,300 | 192,300 |
| GM/GS-15 | 22 | 23 | 23 |
| GM/GS-14 | 22 | 25 | 25 |
| GM/GS-13 | 19 | 23 | 23 |
| GS-12 | 17 | 18 | 18 |
| GS-11 | 4 | 5 | 5 |
| GS-10 | 0 | 0 | 0 |
| GS-9 | 8 | 8 | 8 |
| GS-8 | 5 | 5 | 5 |
| GS-7 | 0 | 0 | 0 |
| GS-6 | 0 | 0 | 0 |
| GS-5 | 0 | 0 | 0 |
| GS-4 | 2 | 2 | 2 |
| GS-3 | 1 | 1 | 1 |
| GS-2 | 1 | 1 | 1 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 101 | 111 | 111 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): | |||
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 0 | 0 | 0 |
| Senior Grade | 1 | 1 | 1 |
| Full Grade | 0 | 0 | 0 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 1 | 1 | 1 |
| Ungraded | 45 | 45 | 45 |
| Total permanent positions | 99 | 110 | 110 |
| Total positions, end of year | 148 | 158 | 158 |
| Total full-time equivalent (FTE) employment, end of year | 129 | 140 | 140 |
| Average ES salary | 192,254 | 192,300 | 192,300 |
| Average GM/GS grade | 12.4 | 12.4 | 12.4 |
| Average GM/GS salary | 118,249 | 121,918 | 122,869 |
-
www.nidcd.nih.gov/about/strategic-plan/2017-2021/introduction
-
www.nidcd.nih.gov/health/statistics/quick-statistics-hearing
-
www.nidcd.nih.gov/news/2016/more-1-20-us-children-have-dizziness-and-balance-problems
-
www.nidcd.nih.gov/health/statistics/quick-statistics-hearing#7
-
www.nidcd.nih.gov/about/strategic-plan/2017-2021/priority-areas-hearing-and-balance-research
-
NIDCD Epidemiology and Statistics Branch calculation
-
www.ncbi.nlm.nih.gov/pubmed/18695010: www.ncbi.nlm.nih.gov/pubmed/17883441: www.ncbi.nlm.nih.gov/pubmed/15679523
-
www.grants.nih.gov/grants/guide/rfa-files/rfa-mh-19-120.html
-
www.grants.nih.gov/grants/guide/rfa-files/RFA-MH-19-121.html
-
https://projectreporter.nih.gov/Reporter_Viewsh.cfm?sl=14E8C10E4A89CFD37598B8961CAA4A01A2FFCEB861BF
* Note: PDF files require a viewer such as the free Adobe Reader.

