 On August 18, 2017, President Trump signed H.R. 2430, the FDA Reauthorization Act, into law (PL 115-52). This law directs the U.S. Food and Drug Administration (FDA) to create a category of over-the-counter (OTC) hearing aids for adults with perceived mild-to-moderate hearing loss. This is a significant step toward improving hearing health care for millions of Americans who have hearing loss by making hearing aids more accessible and affordable.
On August 18, 2017, President Trump signed H.R. 2430, the FDA Reauthorization Act, into law (PL 115-52). This law directs the U.S. Food and Drug Administration (FDA) to create a category of over-the-counter (OTC) hearing aids for adults with perceived mild-to-moderate hearing loss. This is a significant step toward improving hearing health care for millions of Americans who have hearing loss by making hearing aids more accessible and affordable.
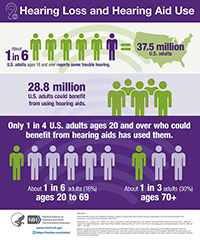
Approximately 28.8 million adults in the U.S. could benefit from using hearing aids, yet only one in four has ever used one. Hearing loss often leads to isolation, and has been associated with serious conditions such as depression, anxiety, low self-esteem, dementia, reduced mobility, and falls. Hearing health care advocates hope that the availability of OTC hearing aids will make it easier for adults with perceived mild-to-moderate hearing loss to benefit from hearing aids. With the help of effective hearing devices, people with hearing loss can again enjoy socializing with family and friends, and they will be less likely to miss important instructions from health providers or safety officials.
The National Institute on Deafness and Other Communication Disorders (NIDCD) has a long history of research and discovery in hearing health. We support studies not only on how we hear, but also on technologies to help people hear better and on delivery models to get hearing loss interventions into the hands of the people who need them.
We were proud to support a recent consensus study by the National Academies of Sciences, Engineering, and Medicine (NASEM) on “Hearing Health Care for Adults: Priorities for Improving Access and Affordability.” Cosponsored by the National Institute on Aging, four other federal agencies, and a nonprofit patient advocacy group, the study concluded that the diverse needs of adults with hearing loss were not being met. One of the independent panel’s 12 recommendations to improve hearing health care is for the FDA to implement a new category of OTC hearing devices. H.R. 2430 directs the FDA to establish new guidelines and quality standards within the next three years. The law was passed just 14 months after the NASEM panel released its report.
The NIDCD is committed to exploring solutions to improve hearing health care for Americans. We continue to support a broad range of hearing health research, including dozens of clinical and translational research projects to develop and test novel strategies to overcome barriers to care and to improve quality of life for the millions of American adults with hearing loss.

