Imagine children playing a game of Red Rover on the playground. Children on one team form a line and hold hands firmly. The other team’s goal is to send a friend to run over and break through the line. Sometimes a wily child gets through, but often nobody does. The human body plays a similar game. The surfaces of our organs, blood vessels, brain, lungs, skin, and heart have a layer of firmly but flexibly connected epithelial cells. The children forming the line are like a layer of epithelial cells, and their hand-holding mimics the connections between cells that prevent most particles from passing through.
The properties of the connections between these cells are important in both determining how well the cell layer can avoid particles crossing and in making the cell layer flexible. This flexibility is particularly important when the cells need to shift around to remove old cells or incorporate new cells.
A study published in Nature Communications on October 18, 2017, describes a new way to peer into the protective connections, or junctions, in animal cell cultures to directly measure their properties. The research was supported by the intramural divisions of the National Institute on Deafness and Other Communication Disorders (NIDCD) and the National Heart, Lung, and Blood Institute (NHLBI), both part of the National Institutes of Health (NIH).
Until now, scientists lacked effective ways to directly measure the junctions between epithelial cells; previous methods were too crude and required damaging the cells in the process. In this study, the researchers modified a method called non-contact acoustic frequency-modulation atomic force microscopy (FM-AFM). With FM-AFM, a tiny probe is brought close to the cells and gently vibrates, not causing any damage to the cells. The researchers here created a new theory so they could use this method to study previously unmeasurable properties and forces between cells. Since conducting the research described in this study, the researchers have also used FM-AFM to image the landscape of the cell layer.
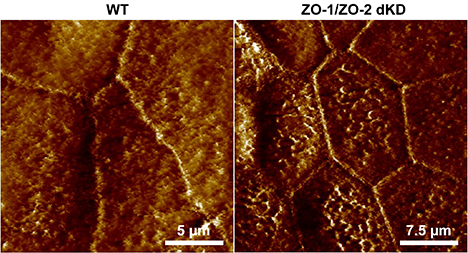
Specifically, the researchers compared normal cells to cells lacking proteins called ZO-1 and ZO-2. These proteins are important for a kind of junction between cells called a tight junction. Previous researchers had noticed that removing these proteins likely caused an increase in tension across the epithelial cell layer. Using this new version of FM-AFM, researchers could measure the increase in tension; moreover, using these tension measurements together with information about the hexagonal shape of the cells, they could explore the forces between epithelial cells.
Junctions between epithelial cells are important for our senses and brain to work properly. “The molecules and forces they exert are in all epithelial cells, including sensory epithelia,” says Richard Chadwick, Ph.D., chief of the Section on Auditory Mechanics at the NIDCD, and the corresponding author of this paper. “So this research is extremely relevant to the hearing and balance organs.” For example, the forces between epithelial cells change in the inner ear throughout development. “These junctions are likely to play a critical role in directing how hearing and balance structures are formed,” Chadwick adds.
This study was supported by the NIH’s NIDCD (ZIA-DC00003321) and NHLBI (ZIA-HL006207).

