- Download Congressional Justification 2024 (1.42MB PDF)
FY 2024 Budget
DEPARTMENT OF HEALTH AND HUMAN SERVICES
NATIONAL INSTITUTES OF HEALTH
National Institute on Deafness and Other Communication Disorders
On this page:
- Director’s Overview
- NIDCD Fact Sheet
- Major Changes in the Budget Request
- Budget Mechanism Table
- Appropriations Language
- Summary of Changes
- Budget Graphs
- Organization Chart
- Budget Authority by Activity Table
- Justification of Budget Request
- Appropriations History
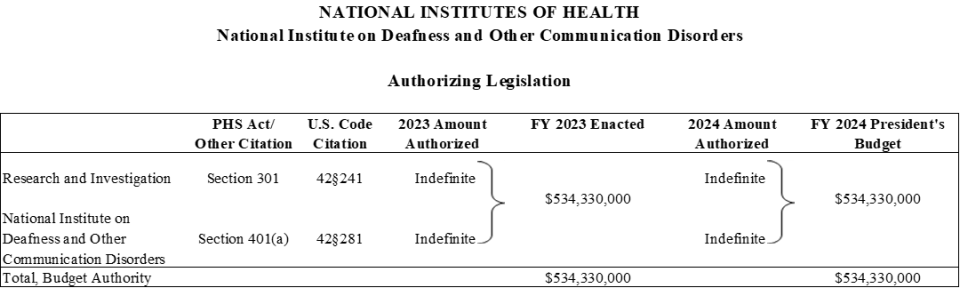
- Authorizing Legislation
- Amounts Available for Obligation
- Budget Authority by Object Class
- Salaries and Expenses
- Detail of Full-Time Equivalent Employment (FTE)
- Detail of Positions
General Notes
- FY 2023 Enacted levels cited in this document include the effects of the FY 2023 HIV/AIDS transfer, as shown in the Amounts Available for Obligation table.
- Detail in this document may not sum to the subtotals and totals due to rounding.
Director’s Overview

M.D., M.S., M.B.A.
Director, NIDCD
About one in six Americans have a hearing, balance, sensory, or other communication disorder. For these individuals, the basic components of communication—sensing, interpreting, and responding to people and things in our environment—can be challenging. The National Institute on Deafness and Other Communication Disorders (NIDCD) manages a broad portfolio of basic, translational, clinical, and public health research focused on human communication and associated disorders in three program areas: hearing and balance; taste and smell; and voice, speech, and language. NIDCD’s vision is to advance the science of communication to improve lives.
Promoting the public good: In August 2022, the United States marked a significant milestone in advancing hearing health care, as the Food and Drug Administration (FDA) issued its final rule establishing guidelines for manufacturing a new class of hearing aids that can be sold over the counter (OTC). This landmark ruling is part of a broader effort to expand access to high-quality, affordable hearing health care for all Americans. OTC hearing aids are intended to address perceived mild to moderate hearing loss in people 18 or older. Now, millions of adults who have trouble hearing will be able to buy less expensive hearing aids without a medical exam, prescription, or fitting by an audiologist or other licensed provider.
The new regulation is the culmination of decades of research efforts by NIDCD and others exploring key issues faced by people with hearing loss. In the United States alone, this serious and often overlooked public health issue affects an estimated 30 million people. Hearing loss is associated with an increased risk of dementia, falls, depression, social isolation, anxiety, and reduced mobility. In order to address this urgent public health need, NIDCD supports innovative clinical and translational research initiatives that lay the foundation for improving accessible and affordable hearing health care, such as identifying barriers to hearing health care, assessing novel service delivery and screening models, and other approaches to improve access to and affordability of hearing health care for adults.1
Ensuring health at all stages of life for all people: Communication disorders, including developmental language disorder, autism spectrum disorder, and hearing loss, are among the most prevalent disabilities in early childhood. These conditions may cause long-term social, emotional, behavioral, academic, physical, and mental health risks in children. Early intervention (EI) for these disorders can improve outcomes. However, children from racial, ethnic, and linguistic minority populations, including those living in rural areas or in poverty, have less access to timely, accurate identification or high-quality treatment for communication disorders. To address this disparity, NIDCD-supported scientists are conducting a study to identify key sources of structural racism and discrimination for children with communication disorders in the Individuals with Disabilities Education Act Part C Early Intervention system.2
The researchers are engaging parents of children of color with disabilities or who speak non-English languages, pediatricians, speech-language pathologists, EI developmental specialists, EI leadership, and scholars; and the researchers are using data from over 100,000 children in five states (Oregon, Indiana, Minnesota, Nevada, and Florida). The study will apply a health disparities framework to precisely detect, understand, and develop solutions that address sources of structural racism and discrimination in EI at the individual, organizational, and community levels. Researchers will use the findings to develop a set of comprehensive, data-driven, and feasible recommendations to improve equity in early care for children of color affected by communication disorders.
Nonsyndromic hearing impairment (NSHI) is a partial or total loss of hearing that is not associated with other symptoms. Researchers have identified almost 125 genes that are associated with NSHI. Many of these genes are involved in the development and function of the inner ear. To date, most NSHI gene identification studies have been performed in families from Europe and Asia, with the greatest number of findings in NSHI families from the Middle East and the Indian Subcontinent. To acquire a complete picture of the genes and variants involved in NSHI, gene identification needs to be performed in additional populations, in particular sub-Saharan Africans. Additionally, NSHI is understudied in African-Americans and little is known about the genetic cause of NSHI in this population. NIDCD-supported scientists are conducting the largest study of NSHI to date in sub-Saharan Africans using exome and whole genome sequencing and bioinformatic evaluation to find genes containing NSHI gene mutations.3 The results of this study will improve genetic screening, and in the future, may improve prediction of treatment outcomes in sub-Saharan Africans, African Americans, and Hispanic Americans of African descent.
Approximately 1 in 10 adults experience tinnitus, and among them about 27 percent report symptoms lasting longer than 15 years.4 Individuals with tinnitus may hear unwanted sounds such as ringing or buzzing. Many aspects of daily life, such as sleep, mood, and concentration, may be affected as a result of having tinnitus, for which there is no cure. Specialized, clinical services for tinnitus are needed but may not be readily available. An effective approach to managing tinnitus is using cognitive behavioral therapy (CBT), which aims to reduce or correct one’s negative response to that perception. NIDCD-supported scientists are developing a solution to make CBT treatment of tinnitus widely available by offering CBT via the internet (iCBT) as a guided self-help program and customizing it to meet individual needs both in terms of language (English or Spanish) and time of usage.5 Results indicate that the iCBT platform helps individuals with tinnitus self-manage their coping and adaptation to reduce tinnitus-related distress and insomnia. This preliminary trial is the first study to evaluate and indicate the efficacy of audiologist-delivered internet-based CBT in reducing tinnitus distress in a U.S. population and to offer this type of tinnitus program in Spanish to accommodate the large Hispanic population in the United States.
Inspiring the next generation of scientists: Diverse research teams are more likely to ensure that members of underserved populations will support and participate in research and that the research addresses questions that are meaningful to these communities. NIDCD strives to increase the diversity of the research pipeline across our mission areas through a variety of mechanisms. For example, NIDCD funds a program titled ‘Research Opportunities for New Investigators to Promote Workforce Diversity.’6 The objective of this program is consistent with the provisions of the 21st Century Cures Act and supports research from new investigators from minority and underrepresented minority racial and ethnic groups proposing independent research projects related to sensory and communication sciences.

NIDCD uses the Maximizing Opportunities for Scientific and Academic Independent Careers (MOSAIC) K99/R007 program to support promising postdoctoral investigators from diverse backgrounds who are conducting research in NIDCD research areas and who are ready to complete the transition from postdoctoral researchers to independent investigators. The MOSAIC program offers scholars a variety of development activities, such as lab management, grants management, scientific publishing training, mentoring and networking, and career visibility and enhancement training. With professional skills development and introductions into mentoring and professional networks, MOSAIC scholars are equipped to transition into, advance, and succeed in independent, tenure-track or equivalent research-intensive faculty careers.
At the organization level, NIDCD provides administrative supplemental funding to specific types of research grants to enhance the participation of individuals from groups that are underrepresented in NIDCD-specific mission areas. Supplements provide support for research experiences for individuals throughout the continuum from high school to the faculty level by recruiting and supporting students, postdoctoral fellows, and eligible investigators, including individuals from diverse backgrounds and from groups that have been shown to be underrepresented in health-related research. This funding opportunity is also available to investigators of research grants who are or become disabled and need additional support to accommodate their disability. NIDCD’s continued outreach of the administrative supplements program has generated a significant response from the research community. In addition, NIDCD has established the NIDCD Diversity Scholars Program, which recognizes the engagement and research commitment of a diverse and accomplished group of scholars and mentors. NIDCD hosted a virtual bootcamp in June 2022 to provide the Diversity Scholars with an overview of NIH and an opportunity for them to ask questions through an interactive session.
Developing targeted preventions and cures: NIDCD has been able to expand support for innovative clinical trials that may one day lead to prevention strategies and treatments that improve the quality of life for individuals who have hearing loss or communication disorders. Examples of NIDCD-supported clinical trials are listed below.
Improving clinical outcomes for cochlear implant users: A cochlear implant is a small, complex electronic device that provides a sense of sound to a person with significant or total hearing loss. Candidacy criteria for cochlear implantation has expanded in recent years to include individuals with more residual hearing (the ability to hear some sounds even though significant hearing loss exists); however, one major challenge is preserving the residual hearing, which can be lost following implant surgery. NIDCD supports a multicenter clinical trial examining how an existing diagnostic technology, called electrocochleography, can be used to help guide implant placement in the inner ear to avoid tissue injury.8 The study will also investigate if combined hearing aid and cochlear implant technology in the same ear is a better treatment option than only cochlear implantation for individuals with sufficient residual hearing. The results of this trial will inform evidence-based clinical practice to improve hearing outcomes for a growing population of individuals receiving cochlear implants.
Diet-induced modification of sweet-taste preference: NIDCD also supports a clinical trial to try to change how humans perceive and prefer sweet tastes.9 A diet rich in sugar intake is known to cause poor health outcomes. The natural human tendency to seek out sweet tastes is challenging, and this trial is modeled on previous work on salt taste, which showed that consuming a low salt diet gradually led people to prefer less salt in their foods. The current trial takes a similar approach, in that people will eat less dietary sugar, and the investigators will determine whether participants gradually prefer less sugar in their diets over the course of a three-month period. If this study establishes that reduced sugar consumption is a valid way to reduce preference for sugar in our diets, it may inform future studies that provide evidence for policy recommendations concerning reduction of simple sugars in manufactured and prepared foods to provide a healthier diet for everyone.
Interventions for preschool children with language disorders: Language acquisition is the process by which children acquire the ability to perceive and understand language, as well as the ability to produce and use words and sentences to communicate. Developmental language disorder (DLD) is a communication disorder that interferes with the development of language skills in children who have no hearing loss or intellectual disabilities. It is one of the most common developmental disorders, affecting approximately seven percent of children in kindergarten.10 Childhood language disorders pose significant risk for poor academic, behavioral, and social outcomes across the school years and into adulthood. NIDCD-supported researchers are conducting a Phase II clinical trial that evaluates the efficacy of an 18-month parent-implemented intervention for accelerating language growth and reducing risk for DLD in preschool children with language delays.11 This study will determine if sustained early intervention can reduce the long-term risks of DLD, inform developmental theory, and provide critical information that may transform preschool service delivery models.
NIDCD Fact Sheet
Advancing the science of communication to improve lives

M.D., M.S., M.B.A.
Director, NIDCD
For over 30 years, NIDCD’s focus has been to bring national attention to normal and disordered processes of hearing, balance, taste, smell, voice, speech, and language and to contribute to advances in biomedical and behavioral research that will improve the lives of the tens of millions of Americans with deafness and communication disorders. NIDCD employs a wide range of research approaches such as molecular genetics, biomedical imaging, cellular biology, nanotechnology, psychoacoustics, and structural and functional biology to advance the development of better diagnostics, prevention strategies, and treatments.
Appropriations History
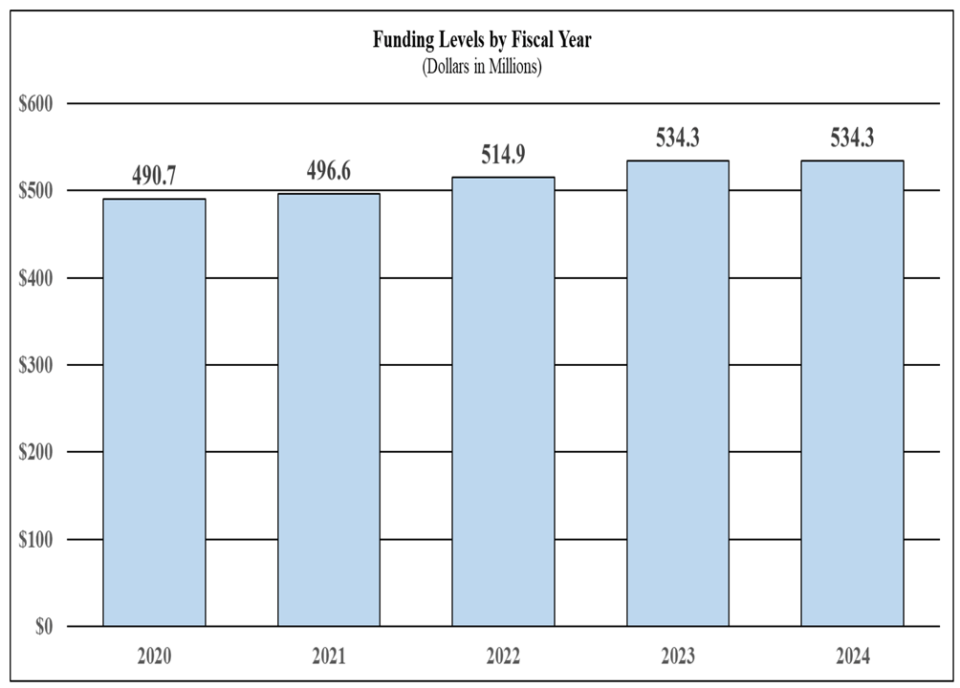
President’s Budget FY 2024: $534,330,000
FY 2023: $534,330,000
FY 2022: $514,882,000
FY 2021: $496,578,000
FY 2020: $490,692,000
FY 2019: $474,404,000
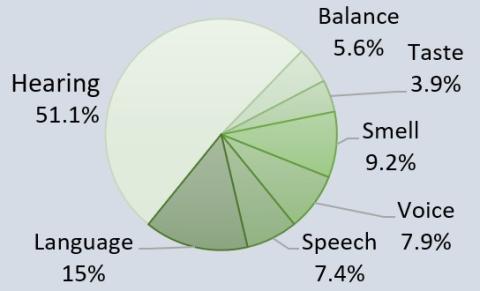
Funding by Mission Area (FY 2022)
Research on Long COVID

NIDCD researchers will use mouse models of COVID-19 to investigate the long-term taste loss caused by COVID-19 and the extent of taste bud tissue damage due to SARS-CoV-2 infection. Another group will use human olfactory epithelium biopsies (tissue from the lining of the nose) to study how the olfactory epithelium is affected by SARS-CoV-2 infection. Researchers will compare olfactory biopsies from COVID-19 patients who have recovered olfactory function, have persistent smell loss, or have developed persistent parosmia, a smell distortion. Findings from these research projects will be informative to better understand taste and smell loss in general as well as loss associated with COVID-19.
FY 2022 Facts & Figures
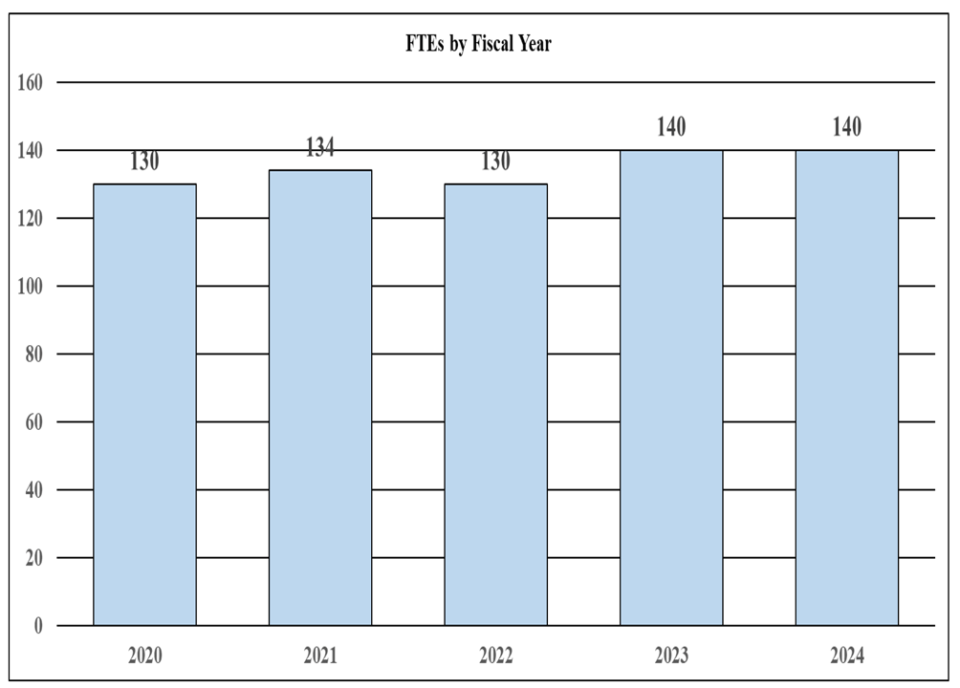
- 130 Full Time Equivalent Employees
- 230 Principal Investigators
- 24.1% Success Rate (R01)
- 30.8% Early-Stage Investigator Success Rate (R01)
- 191 Competing Research Project Grants
- 66 Competing Individual and Institutional Training Awards
Diagnosing Hidden Hearing Loss

NIDCD supports research to improve ways in which clinicians diagnose hearing loss. Standard hearing tests measure how well people detect certain frequencies of sound. Yet some people who have normal results on standard hearing tests may still struggle to follow a conversation in noisy environments. This type of hearing loss is sometimes called “hidden hearing loss” because it isn’t readily detected using common tests of hearing. Researchers developed a method, based on word recognition, to estimate the loss of nerve functioning in the inner ear. With further development, the method could be used in hearing exams to determine whether hearing problems are due to nerve fiber loss.
Health Disparities and Inequities Research

For decades, researchers have documented inequalities in health and healthcare in racial and ethnic minority, socioeconomically disadvantaged, sexual and gender minority, and rural populations. Further, there has been limited effort to date to systematically explore health disparities, including issues of racism and discrimination, in communication disorders among underrepresented groups or to resolve these disparities and promote equity in care. Sufficient evidence exists to affirm that underrepresented minorities’ communication disorders have been — and continue to be — understudied and underserved. Therefore, NIDCD encourages research to advance the understanding or reduce the impact of health disparities and inequities in communication disorders.
2023–2027 NIDCD Strategic Plan
With guidance from our advisory councils and input from our scientific community, NIDCD evaluated our current research portfolios and priorities and examined the impact of NIDCD-related disorders and conditions on public health. The result was the 2023–2027 NIDCD Strategic Plan to guide research investments with a vision toward the future. Similarly, NIDCD’s Division of Intramural Research developed a strategic plan to set priorities in research and NIH-wide collaborations. Both plans aim to advance NIDCD strategic objectives to support innovative, crosscutting, multi-disciplinary research so all Americans can benefit from scientific discovery that will lead to effective and accessible prevention strategies and treatments for hearing loss and communication disorders.
Major Changes in the Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2024 President’s Budget request for NIDCD, which is the same as the FY 2023 Enacted level of $534.3 million.
Other Research (+$0.9 million; total $20.6 million):
NIDCD will fund 114 Other Research awards in FY 2024, an increase of 5 awards from the FY 2023 Enacted level.
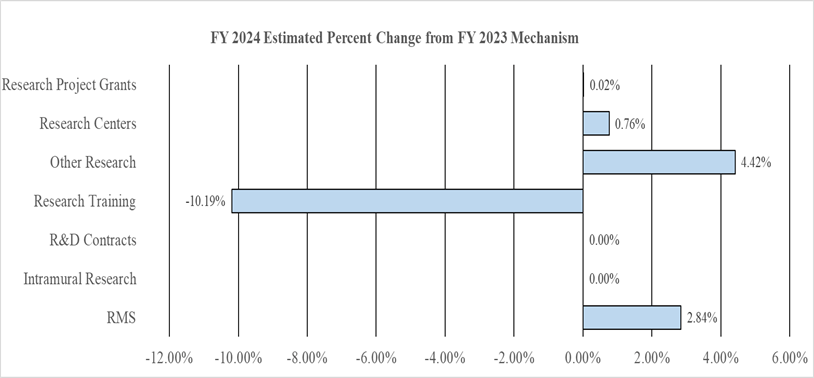
Research Training (-$1.8 million; total $16.0 million):
NIDCD will fund 314 training awards in FY 2024, an increase of 4 from the FY 2023 Enacted level. The decrease in funding is due to overlaps between Institutional Training and growth in NIDCD’s Other Research program.
| (Dollars in Thousands) | ||||||||
| Mechanism | FY 2022 Final | FY 2023 Enacted | FY 2024 President's Budget | FY 2024 +/- FY 2023 | ||||
|---|---|---|---|---|---|---|---|---|
| Number | Amount | Number | Amount | Number | Amount | Number | Amount | |
| Research Projects: | ||||||||
| Noncompeting | 581 | $264,766 | 582 | $268,925 | 585 | $270,500 | 3 | $1,575 |
| Administrative Supplements | (71) | $5,560 | (65) | $5,000 | (52) | $4,000 | -(13) | -$1,000 |
| Competing: | ||||||||
| Renewal | 38 | $20,486 | 38 | $20,311 | 37 | $20,250 | -1 | -$61 |
| New | 153 | $59,857 | 155 | $60,933 | 154 | $60,475 | -1 | -$458 |
| Supplements | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Subtotal, Competing | 191 | $80,343 | 193 | $81,244 | 191 | $80,725 | -2 | -$519 |
| Subtotal, RPGs | 772 | $350,669 | 775 | $355,169 | 776 | $355,225 | 1 | $56 |
| SBIR/STTR | 27 | $16,015 | 27 | $16,300 | 27 | $16,300 | 0 | $0 |
| Research Project Grants | 799 | $366,683 | 802 | $371,469 | 803 | $371,525 | 1 | $56 |
| Research Centers: | ||||||||
| Specialized/Comprehensive | 6 | $15,318 | 7 | $18,434 | 7 | $18,574 | 0 | $140 |
| Clinical Research | 0 | $0 | 0 | $31 | 0 | $31 | 0 | $0 |
| Biotechnology | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Comparative Medicine | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Research Centers in Minority Institutions | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Research Centers | 6 | $15,318 | 7 | $18,465 | 7 | $18,605 | 0 | $140 |
| Other Research: | ||||||||
| Research Careers | 58 | $8,884 | 67 | $10,746 | 68 | $10,900 | 1 | $154 |
| Cancer Education | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Cooperative Clinical Research | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Biomedical Research Support | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Minority Biomedical Research Support | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Other | 33 | $7,298 | 42 | $8,935 | 46 | $9,650 | 4 | $715 |
| Other Research | 91 | $16,182 | 109 | $19,681 | 114 | $20,550 | 5 | $869 |
| Total Research Grants | 896 | $398,183 | 918 | $409,615 | 924 | $410,680 | 6 | $1,065 |
| Ruth L Kirschstein Training Awards: | FTTPs | FTTPs | FTTPs | FTTPs | ||||
| Individual Awards | 142 | $6,997 | 156 | $7,854 | 160 | $8,000 | 4 | $146 |
| Institutional Awards | 153 | $9,631 | 154 | $9,961 | 154 | $8,000 | 0 | -$1,961 |
| Total Research Training | 295 | $16,628 | 310 | $17,815 | 314 | $16,000 | 4 | -$1,815 |
| Research & Develop. Contracts | 44 | $23,229 | 44 | $23,500 | 44 | $23,500 | 0 | $0 |
| (SBIR/STTR) (non-add) | (0) | ($204) | (0) | ($200) | (0) | ($0) | (0) | -($200) |
| Intramural Research | 56 | $52,588 | 64 | $57,000 | 64 | $57,000 | 0 | $0 |
| Res. Management & Support | 74 | $24,254 | 76 | $26,400 | 76 | $27,150 | 0 | $750 |
| SBIR Admin. (non-add) | ($0) | ($0) | ($0) | ($0) | ||||
| Construction | $0 | $0 | $0 | $0 | ||||
| Buildings and Facilities | $0 | $0 | $0 | $0 | ||||
| Total, NIDCD | 130 | $514,882 | 140 | $534,330 | 140 | $534,330 | 0 | $0 |
| *All items in italics and brackets are non-add entries. | ||||||||
Appropriations Language
NATIONAL INSTITUTES OF HEALTH
NATIONAL INSTITUTE ON DEAFNESS AND OTHER COMMUNICATION DISORDERS
For carrying out section 301 and title IV of the PHS Act with respect to deafness and other communication disorders, [$534,333,000]$534,330,000.
| (Dollars in Thousands) | ||||||
| FY 2023 Enacted | $534,330 | |||||
| FY 2024 President's Budget | $534,330 | |||||
| Net change | $0 | |||||
| CHANGES | FY 2023 Enacted | FY 2024 President's Budget | Built-In Change from FY 2023 Enacted | |||
|---|---|---|---|---|---|---|
| FTEs | Budget Authority | FTEs | Budget Authority | FTEs | Budget Authority | |
| A. Built-in: | ||||||
| 1. Intramural Research: | ||||||
| a. Annualization of FY 2023 pay and benefits increase | $13,224 | $13,931 | $147 | |||
| b. FY 2024 pay and benefits increase | $13,224 | $13,931 | $507 | |||
| c. Paid days adjustment | $13,224 | $13,931 | $51 | |||
| d. Differences attributable to change in FTE | $13,224 | $13,931 | $0 | |||
| e. Payment for centrally furnished services | $7,466 | $7,585 | $119 | |||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | $36,310 | $35,484 | $815 | |||
| Subtotal | $1,639 | |||||
| 2. Research Management and Support: | ||||||
| a. Annualization of FY 2023 pay and benefits increase | $16,641 | $17,530 | $184 | |||
| b. FY 2024 pay and benefits increase | $16,641 | $17,530 | $637 | |||
| c. Paid days adjustment | $16,641 | $17,530 | $64 | |||
| d. Differences attributable to change in FTE | $16,641 | $17,530 | $0 | |||
| e. Payment for centrally furnished services | $1,666 | $1,693 | $27 | |||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | $8,093 | $7,928 | $166 | |||
| Subtotal | $1,078 | |||||
| Subtotal, Built-in | $2,717 | |||||
| CHANGES | FY 2023 Enacted | FY 2024 President's Budget | Program Change from FY 2023 Enacted | |||
|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | |
| B. Program: | ||||||
| 1. Research Project Grants: | ||||||
| a. Noncompeting | 582 | $273,925 | 585 | $274,500 | 3 | $575 |
| b. Competing | 193 | $81,244 | 191 | $80,725 | -2 | -$519 |
| c. SBIR/STTR | 27 | $16,300 | 27 | $16,300 | 0 | $0 |
| Subtotal, RPGs | 802 | $371,469 | 803 | $371,525 | 1 | $56 |
| 2. Research Centers | 7 | $18,465 | 7 | $18,605 | 0 | $140 |
| 3. Other Research | 109 | $19,681 | 114 | $20,550 | 5 | $869 |
| 4. Research Training | 310 | $17,815 | 314 | $16,000 | 4 | -$1,815 |
| 5. Research and development contracts | 44 | $23,500 | 44 | $23,500 | 0 | $0 |
| Subtotal, Extramural | $450,930 | $450,180 | -$750 | |||
| 6. Intramural Research | 64 | $57,000 | 64 | $57,000 | 0 | -$1,639 |
| 7. Research Management and Support | 76 | $26,400 | 76 | $27,150 | 0 | -$328 |
| 8. Construction | $0 | $0 | $0 | |||
| 9. Buildings and Facilities | $0 | $0 | $0 | |||
| Subtotal, Program | 140 | $534,330 | 140 | $534,330 | 0 | -$2,717 |
| Total built-in and program changes | $0 | |||||
Budget Graphs
Fiscal Year 2024 Budget Graphs
History of Budget Authority and FTEs:
Distribution by Mechanism:
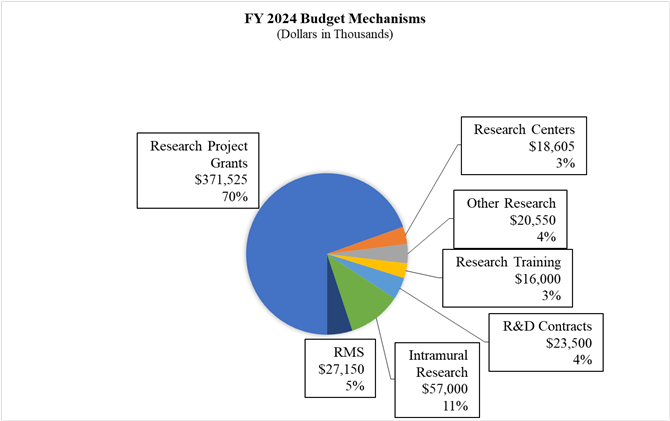
Change by Selected Mechanisms:
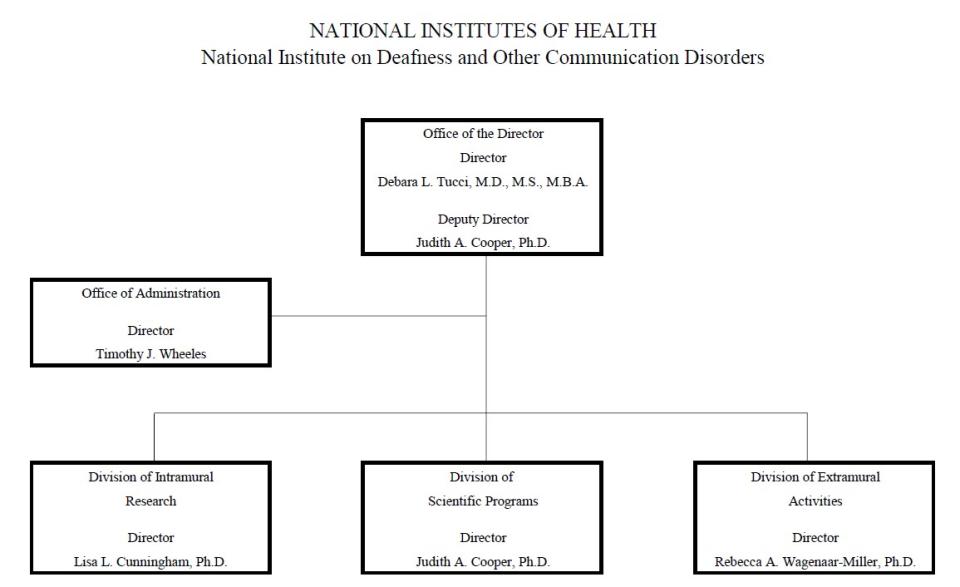
Organization Chart
| (Dollars in Thousands) | ||||||||
|---|---|---|---|---|---|---|---|---|
| FY 2022 Final | FY 2023 Enacted | FY 2024 President's Budget | FY 2024 +/- FY 2023 Enacted | |||||
| Extramural Research | FTE | Amount | FTE | Amount | FTE | Amount | FTE | Amount |
| Detail | ||||||||
| Hearing and Balance | $232,683 | $239,530 | $239,132 | -$398 | ||||
| Taste and Smell | $61,911 | $63,733 | $63,627 | -$106 | ||||
| Voice, Speech, and Language | $143,446 | $147,667 | $147,422 | -$246 | ||||
| Subtotal, Extramural | $438,040 | $450,930 | $450,180 | -$750 | ||||
| Intramural Research | 56 | $52,588 | 64 | $57,000 | 64 | $57,000 | 0 | $0 |
| Research Management & Support | 74 | $24,254 | 76 | $26,400 | 76 | $27,150 | 0 | $750 |
| TOTAL | 130 | $514,882 | 140 | $534,330 | 140 | $534,330 | 0 | $0 |
| *Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | ||||||||
| FY 2022 Final | FY 2023 Enacted | FY 2024 President's Budget | FY 2024 +/- FY 2023 | |
|---|---|---|---|---|
| BA | $514,882,000 | $534,330,000 | $534,330,000 | $0 |
| FTE | 130 | 140 | 140 | 0 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Overall Budget Policy: The FY 2024 President’s Budget request for NIDCD is $534.3 million, which is the same as the FY 2023 Enacted level.
Program Descriptions
Hearing and Balance Program
Loss of hearing or balance imposes a significant social and economic burden upon individuals, their families, and the communities in which they live. Hearing and balance disorders cross all ethnic and socioeconomic lines. Among adults, hearing loss is the third most common chronic condition in the United States.12 The prevalence of hearing loss increases with age, with nearly 25 percent of adults aged 65–74 having a disabling hearing loss and up to 50 percent for those who are 75 and older.13 About 2 out of every 1,000 children in the United States are born with a detectable level of hearing loss in one or both ears that can affect their speech, language, social, and cognitive development.14 In addition, more than 1 in 20 American children have a dizziness or balance problem.15 Accordingly, NIDCD supports research that will lead to improved treatments for, and prevention of, hearing and balance disorders. Research projects in hearing and balance encompass over half of NIDCD’s grant portfolio.
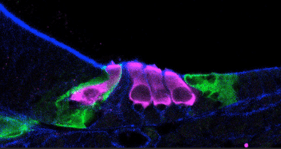
most) and three outer (right most) hair
cells.
Credit: Helen Maunsell, NIDCD/NIH
There are two types of sensory hair cells in the inner ear responsible for hearing. Outer hair cells help amplify sound vibrations entering the inner ear from the middle ear. When hearing is working normally, the inner hair cells convert these vibrations into electrical signals that travel as nerve impulses to the brain, where the brain interprets the impulses as sound. Over our lifetime, these hair cells can be damaged or destroyed by exposure to loud noises, certain medications, physical injury, or normal aging. Hearing loss is difficult to treat because hair cells cannot be repaired or replaced in humans, although they are replaced in other species. NIDCD funds numerous cell-based and animal studies trying to understand the many molecular players that drive hair cell development and replacement in order to regenerate the missing hair cells using such approaches as drugs or gene therapy. For example, one NIDCD-funded research team has discovered a single master gene in mice that programs hair cells into either outer or inner hair cells, overcoming a major hurdle.16 Currently, scientists can produce an artificial hair-like cell, but it does not differentiate into an inner or outer hair cell. The newly discovered master gene TBX2 needs to be turned on or off to produce the needed inner or outer hair cell. While the research is still in early, experimental stages, understanding how these two complementary sensory cell types are generated may one day guide and assist future therapeutic attempts via regeneration.
Neurons that are connected to hair cells begin to atrophy once the cells die and connections to the brain are lost. Basic research studies are also underway to examine how to repair and reform the lost connections between hair cells and the auditory (hearing) or vestibular (balance) nerves. In a recent advance, NIDCD-funded researchers who study zebrafish, which can regenerate its sensory hair cells, investigated how macrophages (a type of white blood cell) can help repair and regenerate a zebrafish sensory organ.17 The scientists discovered that macrophages were important in “cleaning up” the dead hair cells, and they also played an integral role in initiating regeneration and interacting with the brain by communicating with the nerve cells to reestablish and maintain synapses necessary for proper sensory organ function after regeneration. These findings provide invaluable knowledge for designing targeted immunotherapies that may one day be applicable in treating human conditions like hearing loss or other nervous system injury.
Falls are one of the leading causes of accidental death and are the most common and costly safety events in older adults.18 In addition to fractures and other fall-related injuries, falls can lead to disability, risk of hospitalization, activity restriction, and reduction in quality of life. Risk factors for falls in older adults include medications, vision deficits, certain chronic diseases, impaired balance and gait, cognitive impairment, and environmental factors. Evidence also shows hearing loss as being independently associated with falls in older adults. Falls are considered a significant public health issue, and falls risk increases with age. It is unclear if falls can be prevented by treatment of hearing loss with hearing aids or a cochlear implant. NIDCD-supported scientists are conducting a study to identify and understand the mechanisms that may determine the association between falls and hearing loss in an effort to develop interventions that will modify the risk of falls in older adults.19 The primary goal of this study is to determine if hearing-aid use can reduce the association between self-reported hearing ability and falls or falls-related injury. The study will focus on determining whether or not using hearing aids or cochlear implants influences the association between hearing loss and falls and begin to evaluate this associated pathway. If users of hearing aids or cochlear implants have reduced odds of falling compared with non-hearing amplification users, then that would have an important implication for falls prevention.
Tackling the Undiscovered: Nervous System Control of Balance in Older Adults
NIDCD, in conjunction with the National Institute on Aging, supported an initiative to understand the nervous system’s control of balance in older adults. In addition to the hearing organ (cochlea), the inner ear contains the vestibular system, which is responsible for our sense of balance. There are major gaps in our understanding of how aging impacts the vestibular system, which, when impaired, contributes to balance problems and an increased risk of falls in older adults, a significant public health issue. NIDCD is funding a genome-wide association study that will analyze human and mouse genetics and the functional decline of aging vestibular system. The ability to test human genes in animal models allows researchers to identify anatomic and physiologic sites relevant to the balance system. This work has the potential to lay a foundation for the development of targeted treatments for disorders of the vestibular system to diminish falls in older adults.
Mal de debarquement means “sickness of disembarkment” and refers to an illusion of movement felt as an aftereffect of travel. It is an underrecognized yet common balance disorder. Individuals with Mal de Debarquement Syndrome (MdDS) have a constant phantom sensation of motion, even during standing or sleeping. The syndrome is frequently accompanied by increased sensitivity to light, the sensation of ear fullness, head pressure, anxiety, and depression. This disabling condition generally occurs within days after prolonged passive motion (e.g., travel on a cruise ship, plane, train, or a car). NIDCD-supported scientists are studying ways to establish a new effective treatment for MdDS to reduce symptoms immediately and to improve long-term outcomes by reducing the individuals’ susceptibility to recurrent MdDS due to re-exposure to passive motion.20 The scientists hypothesize that MdDS is caused by maladaptation of a functional component of the vestibulo-ocular reflex (VOR) called ‘velocity storage.’ VOR is a reflex that acts to stabilize gaze during head movement with eye movement due to activation of the vestibular system, which provides humans with a sense of balance and information about body position. Velocity storage can be modified (habituated) by training, and scientists are studying whether MdDS symptoms can be improved with habituation training.
Budget Policy: The FY 2024 President’s Budget request for the Hearing and Balance Program is $239.1 million, a decrease of $0.4 million or 0.2 percent compared with the FY 2023 Enacted level.
Taste and Smell Program
Taste and smell disorders are underreported, and often go undiagnosed. NIDCD supports chemosensory research which encompasses taste (gustation), smell (olfaction), and chemesthesis (chemically provoked irritation) studies to help understand how human chemosensory disorders can be diagnosed and treated. The COVID-19 pandemic focused worldwide attention on the impact of the loss of taste and smell because these were common symptoms of infection by the early SARS-CoV-2 viral variants. To explore why smell loss is such a common and persistent symptom of SARS-CoV-2 infection, NIDCD-funded researchers examined nasal tissue from people who died of COVID-19.21 They discovered that the infection caused olfactory sensory neurons to produce fewer receptors that bind odor molecules, which may explain smell loss. The researchers also identified changes in the cell nucleus of olfactory sensory neurons, which resulted in the downregulation of genes encoding proteins important for the binding and detection of odors. These studies inform the understanding of the biological underpinning of how SARS-CoV-2 infection causes smell loss and may provide insight into other neurological consequences of COVID-19.
Tackling the Undiscovered: NIDCD Researchers Aim to Understand Long COVID
The COVID-19 pandemic continues to present considerable public health challenges in the United States and around the globe. One of the most puzzling is why many people who get over an initial and often relatively mild COVID-19 illness later develop new and potentially debilitating symptoms. NIH has created the Researching COVID to Enhance Recovery (RECOVER) Initiative to learn about the long-term effects of COVID-19. Two NIDCD-supported researchers have received funds through the RECOVER Initiative to understand how COVID-19 leads to taste and smell loss and why in some individuals these losses persist. One research team will use mouse models of COVID-19 to investigate the underlying mechanisms of long-term taste loss caused by COVID-19 and the extent of taste bud tissue damage due to SARS-CoV-2 infection. Another group of scientists will use human olfactory epithelium biopsies (tissue from the lining of the nose) to study how the olfactory epithelium is affected by SARS-CoV-2 infection. Researchers will compare olfactory biopsies from COVID-19 patients who have recovered olfactory function, have persistent smell loss, or have developed persistent parosmia, a smell distortion. Findings from these research projects will enhance understanding of taste and smell loss in general, as well as loss associated with COVID-19.
The olfactory system is one of a few areas in the human nervous system which is capable of neuronal regeneration throughout life. Olfactory sensory neurons reside in the nasal cavity and are continuously replenished with new neurons arising from stem cells. With age, the olfactory system gradually loses this ability. NIDCD supports a robust portfolio of research to understand the mechanisms of age-related olfactory loss, or presbyosmia. The olfactory system is directly exposed to the environment, making it vulnerable to accumulated damage. An NIDCD-supported research team examined biopsied human tissue from the lining of the nose, known as the olfactory epithelium (OE).22 They examined the OE and its projections to the brain’s olfactory bulb in a large post-mortem sample of older adults (average age about 70 years). Their analyses identified several possible structural bases for presbyosmia. They counted fewer dividing olfactory stem cells and fewer olfactory sensory neurons, with a corresponding reduction in nerve cell connections with the olfactory bulb. At the same time, they noted increased numbers of cysts and neuromas, or benign tumors, and more OE that lacked olfactory sensory cells. The combination of fewer sensory neurons and connections to the olfactory bulb, less regeneration, and more non-sensory tissue offers an anatomical explanation for age-related smell loss.
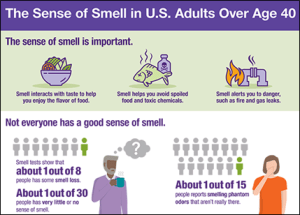
Another NIDCD-supported research team analyzed the biopsies of olfactory tissue from people with presbyosmia.23 They identified high levels of molecules produced in response to stress or inflammation. The team described one molecule, TP63, which prevents OE stem cells from maturing into olfactory sensory neurons. Consequently, the aging olfactory system is less able to replace damaged odor-detecting sensory neurons, offering a possible explanation for presbyosmia. The team’s observations suggest that olfactory stem cells are a potential target for treatments to prevent age-related smell loss. NIDCD encourages further studies of age-related decline in olfactory sensitivity, including the development of better diagnostic tests to assess chemosensory loss, and use of model systems to further elaborate our understanding of why humans experience presbyosmia and how to prevent or treat it.
Budget Policy: The FY 2024 President’s Budget request for the Taste and Smell Program is $63.6 million, a decrease of $0.1 million or 0.2 percent compared with the FY 2023 Enacted level.
Voice, Speech, and Language Program
Disorders involving voice, speech, or language can have an overwhelming effect on an individual’s health and quality of life. These disorders affect people of all ages with or without hearing impairment, including children with autism, those who stutter, and adults with acquired communication disorders. The societal burden of laryngeal disorders is estimated at $11 billion annually due to work-related disability, lost productivity, and direct health care cost.24 By the first grade, roughly five percent of children have noticeable speech disorders that require intervention services.25 In children, delayed speech and language acquisition or impairment are very often significant predictors of future academic, social, vocational, and adaptive outcomes.
NIDCD supports research to understand diseases and stresses that can harm a person’s voice and clinical research to test new ways to diagnose, treat, or cure voice disorders. Regenerative medicine approaches to treat voice disorders that are caused by damage to the laryngeal system, which includes the vocal folds, focus on engineering replacement tissues and methods to regrow nerve connections to repair damaged tissue. One NIDCD-funded research team is developing and testing new biomaterials in animal models that can be injected or deposited layer by layer into a wound site in the larynx to help vocal fold tissue healing and promote the permanent regeneration of engineered tissue with the same look and feel of the original uninjured tissue.26 These materials and tools would help people with vocal fold injuries to recover their voice, with less hoarseness or voice problems. Another study supported by NIDCD aims to determine the molecular signaling pathways that take place during embryologic development that lead to normal nerve connections in the larynx, and then to manipulate these same pathways in an adult animal model of laryngeal tissue injury so that they mimic those in embryology.27 Findings from this research may impact the field of nerve regeneration, advancing scientists’ knowledge of the mechanisms of how nerves make connections with sensory cells and potentially transforming the treatment of voice disorders.
Research on speech perception aims to understand the biology behind how the brain comprehends spoken language. This understanding has significant implications for building computer technology that recognizes speech, improves speech perception for individuals with hearing loss, and allows for the development of new diagnostic and rehabilitative strategies for language disorders. However, the basic mechanisms underlying comprehension of spoken language are still mostly unknown. NIDCD-supported scientists are using an innovative approach to study speech processing by recording neural activity directly from the cortical surface of the brain during neurosurgical procedures.28 The recordings allow researchers to know which parts of the brain detect speech sounds and in what order the information is processed. The overall goal is to determine how the brain extracts linguistic elements from complex speech sounds to one day prevent or treat communication disorders.
Stroke is a leading cause of adult disability in the United States. A significant proportion of stroke survivors have communication disorders, such as aphasia (post-stroke difficulty in using language) as a result of brain injury. Non-fluent aphasia is one of the most common forms of aphasia. Speech is effortful, and most utterances are limited to just a few words. Non-fluent aphasia is directly associated with social isolation, decreased participation in rehabilitation therapies, and low quality of life. NIDCD is supporting a multicenter Phase II clinical trial in people with chronic post-stroke non-fluent aphasia.29 The researchers have optimized a new and promising treatment approach entitled speech entrainment therapy (SET), where the individual’s speech training is guided by an audio-visual model presented on a laptop computer. In a pilot study using SET for treatment of post-stroke non-fluent aphasia, participants spoke more verbs per minute during discourse after six weeks of treatment. This suggests that SET may yield meaningful improvements in fluent spontaneous speech for individuals with non-fluent aphasia.
Budget Policy: The FY 2024 President’s Budget request for the Voice, Speech and Language Program is $147.4 million, a decrease of $0.2 million or 0.2 percent compared with the FY 2023 Enacted level.
Intramural Research Program
The NIDCD Intramural Research Program conducts basic and clinical research in human communication, with a primary focus on hearing and balance.
Developing Targeted Preventions and Cures: Setting and Implementing Strategic Priorities
The NIDCD’s Division of Intramural Research (DIR) held a series of facilitated strategic planning discussions focused on setting priorities, fostering an innovative culture, identifying opportunities for NIH-wide collaboration, and translating basic research into clinical practice. The discussions were guided by the NIDCD Blue Ribbon Panel report and a series of strategic questions. DIR identified the following Strategic Planning priorities:
- Innovation and high-risk, high-reward projects
- Strategic interactions and collaborations
- Resource-building to benefit the entire community
- Data sciences, artificial intelligence and machine learning, and computational power and analyses
- Efficient pipeline for investigators to translate ideas into therapies
- Equity in the scientific workforce
To determine how best to implement the priorities, DIR formed working groups to recommend strategies to achieve the objectives. As a result of those recommendations, DIR has taken the following actions:
- Developed an Innovation Award program to incentivize high-risk, high-impact projects
- Coordinated a neuroscience-focused poster session to foster increased interactions and collaborations
- Obtained funding for and initiated work on an NIH-wide clinical database of hearing and balance function
NIDCD intramural scientists are working to improve cancer treatments, particularly in head and neck cancers that have resisted standard treatments, and which can often lead to communication disorders. One type of cancer immunotherapy uses the body’s own T-cells to eliminate cancer, however some tumor cells can escape T-cell killing. Scientists call these types of cells ‘escape cells.’ NIDCD intramural scientists noted that the escape cells expressed a certain protein on their surface called programmed death ligand-1 (PD-L1), providing an attractive target for developing additional therapies.30 The team used human tumor cells in an animal model and treated the cells with T-cell therapy in combination with engineered human natural killer cells that target PD-L1. This combined therapy succeeded at eliminating the escape cells. The team’s work provides proof of concept evidence to support future clinical trials that combine T-cell-based tumor treatment with natural killer cell therapy for people with head and neck cancer.
Cisplatin is a life-saving cancer drug that can also damage the cells of the inner ear. NIDCD intramural scientists are investigating ways to prevent cisplatin-induced hearing loss in individuals receiving cancer treatment. In a recent advance, the researchers treated mice with cisplatin and then examined changes in specific regions of the inner ear after cisplatin damage.31 They were able to identify changes in gene expression caused by cisplatin, and then the researchers searched a “druggable target” database to predict drugs that might mitigate or reverse the changes in gene expression. In this way, researchers hope to be able to therapeutically target changes prior to the onset of hearing loss as a way to prevent cisplatin-induced hearing loss. Ménière’s disease (MD) is a disorder of the inner ear that causes severe dizziness (vertigo), ringing in the ears (tinnitus), hearing loss, and a feeling of fullness or congestion in the ear. One NIDCD intramural team aims to understand the genetic and cellular basis of MD. A hallmark of MD is enlargement of a structure called the scala media, a fluid containing compartment of the inner ear. On the wall of the scala media is a structure called the stria vascularis. The cells of the stria vascularis are responsible for maintaining appropriate inner ear fluid levels and ion composition, which both play an essential role in normal hearing. The investigators compared published datasets related to MD in humans with previously published adult mouse stria vascularis single-cell datasets to identify genes potentially involved in MD.32 This work will allow for the creation of new genetically engineered mouse models that can provide insight into the underlying mechanisms of MD and aid in identifying future therapeutic targets.
Budget Policy: The FY 2024 President’s Budget request for the Intramural Research Program is $57.0 million, which is equal to the FY 2023 Enacted level.
Research Management and Support (RMS) Program
NIDCD RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS functions also include strategic planning, coordination, and evaluation of the institute’s programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. The institute currently supports approximately 1,400 research grants, training awards, and research and development contracts.
NIDCD developed a new 2023–2027 Strategic Plan which will serve as a guide for how NIDCD will prioritize its research investments as well as outline common goals for the scientific community, advocates, professional organizations, and industry to pursue efforts that will make a significant impact for people who have hearing loss or communication disorders. The Plan presents a series of themes and goals that highlight the most promising research needs within NIDCD’s mission areas as well as NIH-wide crosscutting priorities.
NIDCD is pleased to announce the appointment of NIDCD’s first chief diversity officer. The chief diversity officer reports directly to the NIDCD Director to lead ongoing and future efforts in NIDCD-specific and NIH-wide diversity initiatives. This position provides NIDCD formal scientific leadership and direction for all diversity requirements and initiatives related to NIDCD-supported research. The establishment of a chief diversity officer further strengthens NIDCD’s commitment to create and maintain a culture of inclusiveness at NIDCD.
Budget Policy: The FY 2024 President’s Budget request for RMS is $27.2 million, an increase of $0.8 million or 2.8 percent compared with the FY 2023 Enacted level.
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation | ||||
|---|---|---|---|---|---|---|---|---|
| 2015 | $403,933,000 | $405,302,000 | ||||||
| Rescission | $0 | |||||||
| 2016 | $416,241,000 | $412,366,000 | $424,860,000 | $423,031,000 | ||||
| Rescission | $0 | |||||||
| 20171 | $422,936,000 | $434,126,000 | $441,778,000 | $436,875,000 | ||||
| Rescission | $0 | |||||||
| 2018 | $325,846,000 | $443,624,000 | $451,768,000 | $459,974,000 | ||||
| Rescission | $0 | |||||||
| 2019 | $423,992,000 | $465,467,000 | $474,653,000 | $474,404,000 | ||||
| Rescission | $0 | |||||||
| 2020 | $408,358,000 | $497,590,000 | $500,270,000 | $490,692,000 | ||||
| Rescission | $0 | |||||||
| 2021 | $446,397,000 | $494,912,000 | $506,670,000 | $498,076,000 | ||||
| Rescission | $0 | |||||||
| 2022 | $511,792,000 | $522,758,000 | $511,280,000 | $514,885,000 | ||||
| Rescission | $0 | |||||||
| 2023 | $508,704,000 | $531,136,000 | $530,847,000 | $534,333,000 | ||||
| Rescission | $0 | |||||||
| 2024 | $534,330,000 | |||||||
| 1 Budget Estimate to Congress includes mandatory financing. | ||||||||
Authorizing Legislation
| (Dollars in Thousands) | |||
|---|---|---|---|
| Source of Funding | FY 2022 Final | FY 2023 Enacted | FY 2024 President's Budget |
| Appropriation | $514,885 | $534,333 | $534,330 |
| OAR HIV/AIDS Transfers | -$3 | -$3 | $0 |
| Subtotal, adjusted budget authority | $514,882 | $534,330 | $534,330 |
| Unobligated balance, start of year | $0 | $0 | $0 |
| Unobligated balance, end of year (carryover) | $0 | $0 | $0 |
| Subtotal, adjusted budget authority | $514,882 | $534,330 | $534,330 |
| Unobligated balance lapsing | -$6 | $0 | $0 |
| Total obligations | $514,876 | $534,330 | $534,330 |
| 1 Excludes the following amounts (in thousands) for reimbursable activities carried out by this account: FY 2022 - $1,300 FY 2023 - $2,500 FY 2024 - $2,000 |
|||
| (Dollars in Thousands) | ||||
| FY 2023 Enacted | FY 2024 President's Budget | FY 2024 +/- FY 2023 | ||
|---|---|---|---|---|
| Total compensable workyears: | ||||
| Full-time equivalent | 140 | 140 | 0 | |
| Full-time equivalent of overtime and holiday hours | 0 | 0 | 0 | |
| Average ES salary | $212 | $223 | $11 | |
| Average GM/GS grade | 12.8 | 12.8 | 0.0 | |
| Average GM/GS salary | $133 | $146 | $13 | |
| Average salary, Commissioned Corps (42 U.S.C. 207) | $148 | $156 | $8 | |
| Average salary of ungraded positions | $121 | $128 | $6 | |
| OBJECT CLASSES | FY 2023 Enacted | FY 2024 President's Budget | FY 2024 +/- FY 2023 | |
| Personnel Compensation | ||||
| 11.1 | Full-Time Permanent | $14,919 | $15,733 | $814 |
| 11.3 | Other Than Full-Time Permanent | $5,257 | $5,544 | $287 |
| 11.5 | Other Personnel Compensation | $666 | $702 | $36 |
| 11.7 | Military Personnel | $0 | $0 | $0 |
| 11.8 | Special Personnel Services Payments | $1,552 | $1,637 | $85 |
| 11.9 | Subtotal, Personnel Compensation | $22,394 | $23,615 | $1,221 |
| 12.1 | Civilian Personnel Benefits | $7,471 | $7,845 | $374 |
| 12.2 | Military Personnel Benefits | $0 | $0 | $0 |
| 13.0 | Benefits to Former Personnel | $0 | $0 | $0 |
| Subtotal Pay Costs | $29,865 | $31,461 | $1,596 | |
| 21.0 | Travel & Transportation of Persons | $154 | $158 | $4 |
| 22.0 | Transportation of Things | $64 | $66 | $2 |
| 23.1 | Rental Payments to GSA | $0 | $0 | $0 |
| 23.2 | Rental Payments to Others | $0 | $0 | $0 |
| 23.3 | Communications, Utilities & Misc. Charges | $17 | $17 | $0 |
| 24.0 | Printing & Reproduction | $0 | $0 | $0 |
| 25.1 | Consulting Services | $10,136 | $10,116 | -$20 |
| 25.2 | Other Services | $15,579 | $15,302 | -$278 |
| 25.3 | Purchase of Goods and Services from Government Accounts | $38,950 | $38,634 | -$316 |
| 25.4 | Operation & Maintenance of Facilities | $203 | $203 | $0 |
| 25.5 | R&D Contracts | $3,773 | $3,864 | $91 |
| 25.6 | Medical Care | $437 | $455 | $18 |
| 25.7 | Operation & Maintenance of Equipment | $1,512 | $1,548 | $36 |
| 25.8 | Subsistence & Support of Persons | $0 | $0 | $0 |
| 25.0 | Subtotal Other Contractual Services | $70,590 | $70,121 | -$469 |
| 26.0 | Supplies & Materials | $3,291 | $3,120 | -$171 |
| 31.0 | Equipment | $2,918 | $2,707 | -$211 |
| 32.0 | Land and Structures | $0 | $0 | $0 |
| 33.0 | Investments & Loans | $0 | $0 | $0 |
| 41.0 | Grants, Subsidies & Contributions | $427,430 | $426,680 | -$750 |
| 42.0 | Insurance Claims & Indemnities | $0 | $0 | $0 |
| 43.0 | Interest & Dividends | $1 | $1 | $0 |
| 44.0 | Refunds | $0 | $0 | $0 |
| Subtotal Non-Pay Costs | $504,465 | $502,869 | -$1,596 | |
| Total Budget Authority by Object Class | $534,330 | $534,330 | $0 | |
| 1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | ||||
| (Dollars in Thousands) | |||
| Object Classes | FY 2023 Enacted | FY 2024 President's Budget | FY 2024 +/- FY 2023 |
|---|---|---|---|
| Personnel Compensation | |||
| Full-Time Permanent (11.1) | $14,919 | $15,733 | $814 |
| Other Than Full-Time Permanent (11.3) | $5,257 | $5,544 | $287 |
| Other Personnel Compensation (11.5) | $666 | $702 | $36 |
| Military Personnel (11.7) | $0 | $0 | $0 |
| Special Personnel Services Payments (11.8) | $1,552 | $1,637 | $85 |
| Subtotal, Personnel Compensation (11.9) | $22,394 | $23,615 | $1,221 |
| Civilian Personnel Benefits (12.1) | $7,471 | $7,845 | $374 |
| Military Personnel Benefits (12.2) | $0 | $0 | $0 |
| Benefits to Former Personnel (13.0) | $0 | $0 | $0 |
| Subtotal Pay Costs | $29,865 | $31,461 | $1,596 |
| Travel & Transportation of Persons (21.0) | $154 | $158 | $4 |
| Transportation of Things (22.0) | $64 | $66 | $2 |
| Rental Payments to Others (23.2) | $0 | $0 | $0 |
| Communications, Utilities & Misc. Charges (23.3) | $17 | $17 | $0 |
| Printing & Reproduction (24.0) | $0 | $0 | $0 |
| Other Contractual Services: | |||
| Consultant Services (25.1) | $10,136 | $10,116 | -$20 |
| Other Services (25.2) | $15,579 | $15,302 | -$278 |
| Purchases of Goods and Services from Government Accounts (25.3) | $21,773 | $21,576 | -$197 |
| Operation & Maintenance of Facilities (25.4) | $203 | $203 | $0 |
| Operation & Maintenance of Equipment (25.7) | $1,512 | $1,548 | $36 |
| Subsistence & Support of Persons (25.8) | $0 | $0 | $0 |
| Subtotal Other Contractual Services | $49,203 | $48,745 | -$458 |
| Supplies & Materials (26.0) | $3,291 | $3,120 | -$171 |
| Subtotal Non-Pay Costs | $52,729 | $52,105 | -$623 |
| Total Administrative Costs | $82,594 | $83,566 | $972 |
| Office | FY 2022 Final | FY 2023 Enacted | FY 2024 President's Budget | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Division of Extramural Activities | |||||||||
| Direct: | 19 | - | 19 | 20 | - | 20 | 20 | - | 20 |
| Total: | 19 | - | 19 | 20 | - | 20 | 20 | - | 20 |
| Office of the Director | |||||||||
| Direct: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Total: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Division of Intramural Research Program | |||||||||
| Direct: | 56 | - | 56 | 63 | 1 | 64 | 63 | 1 | 64 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 56 | - | 56 | 63 | 1 | 64 | 63 | 1 | 64 |
| Division of Scientific Programs | |||||||||
| Direct: | 18 | - | 18 | 19 | - | 19 | 19 | - | 19 |
| Total: | 18 | - | 18 | 19 | - | 19 | 19 | - | 19 |
| Office of Administration | |||||||||
| Direct: | 35 | - | 35 | 35 | - | 35 | 35 | - | 35 |
| Total: | 35 | - | 35 | 35 | - | 35 | 35 | - | 35 |
| Total | 130 | - | 130 | 139 | 1 | 140 | 139 | 1 | 140 |
| Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||||||||
| FTEs supported by funds from Cooperative Research and Development Agreements. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FISCAL YEAR | Average GS Grade | ||||||||
| 2020 | 12.4 | ||||||||
| 2021 | 12.6 | ||||||||
| 2022 | 12.8 | ||||||||
| 2023 | 12.8 | ||||||||
| 2024 | 12.8 | ||||||||
| GRADE | FY 2022 Final | FY 2023 Enacted | FY 2024 President's Budget |
|---|---|---|---|
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | $199,300 | $212,100 | $223,129 |
| General Schedule | |||
| GM/GS-15 | 27 | 28 | 28 |
| GM/GS-14 | 20 | 20 | 20 |
| GM/GS-13 | 31 | 32 | 32 |
| GS-12 | 14 | 14 | 14 |
| GS-11 | 3 | 4 | 4 |
| GS-10 | 0 | 0 | 0 |
| GS-9 | 6 | 7 | 7 |
| GS-8 | 3 | 3 | 3 |
| GS-7 | 0 | 1 | 1 |
| GS-6 | 1 | 2 | 2 |
| GS-5 | 2 | 0 | 0 |
| GS-4 | 0 | 0 | 1 |
| GS-3 | 0 | 1 | 0 |
| GS-2 | 1 | 0 | 0 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 108 | 112 | 112 |
| Commissioned Corps (42 U.S.C. 207) | |||
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 0 | 0 | 0 |
| Senior Grade | 1 | 1 | 1 |
| Full Grade | 0 | 0 | 0 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 1 | 1 | 1 |
| Ungraded | 42 | 44 | 44 |
| Total permanent positions | 109 | 115 | 115 |
| Total positions, end of year | 152 | 158 | 158 |
| Total full-time equivalent (FTE) employment, end of year | 130 | 140 | 140 |
| Average ES salary | $199,300 | $212,100 | $223,129 |
| Average GM/GS grade | 12.8 | 12.8 | 12.8 |
| Average GM/GS salary | $132,818 | $132,928 | $146,152 |
| [1] Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||
[32] pubmed.ncbi.nlm.nih.gov/33613436/
*Note: PDF files require a viewer such as the free Adobe Reader.

