- Download Congressional Justification 2019 (504 KB PDF)
FY 2019 Budget
DEPARTMENT OF HEALTH AND HUMAN SERVICES
NATIONAL INSTITUTES OF HEALTH
National Institute on Deafness and Other Communication Disorders (NIDCD)
On this page:
- Organization Chart
- Appropriation Language
- Amounts Available for Obligation
- Budget Graphs
- Authorizing Legislation
- Appropriations History
- Justification of Budget Request
- Detail of Full-Time Equivalent Employment (FTE)
- Detail of Positions
Organization Chart
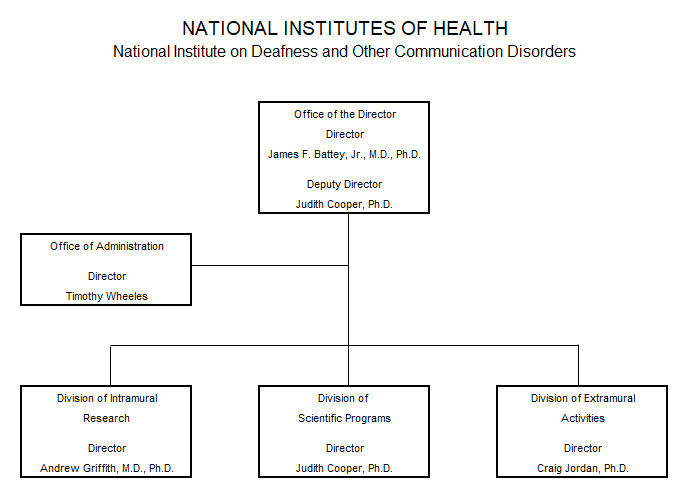
Appropriation Language
NATIONAL INSTITUTES OF HEALTH
National Institute on Deafness and Other Communication Disorders
For carrying out section 301 and title IV of the PHS Act with respect to deafness and other communication disorders, $423,992,000.
| (Dollars in Thousands) | |||
|---|---|---|---|
| Source of Funding | FY 2017 Final | FY 2018 Annualized CR | FY 2019 President's Budget |
| 1 Excludes the following amounts (in thousands) for reimbursable activities carried out by this account: FY 2017 - $1,504; FY 2018 - $2,500; FY 2019 - $2,500 | |||
| Appropriation | $436,875 | $436,875 | $423,992 |
| Mandatory Appropriation: (non-add) | |||
| Type 1 Diabetes | (0) | (0) | (0) |
| Other Mandatory financing | (0) | (0) | (0) |
| Rescission | 0 | -2,967 | 0 |
| Sequestration | 0 | 0 | 0 |
| Secretary's Transfer | -971 | ||
| Subtotal, adjusted appropriation | $435,904 | $433,908 | $423,992 |
| OAR HIV/AIDS Transfers | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | $435,904 | $433,908 | $423,992 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | $435,904 | $433,908 | $423,992 |
| Unobligated balance lapsing | -27 | 0 | 0 |
| Total obligations | $435,877 | $433,908 | $423,992 |
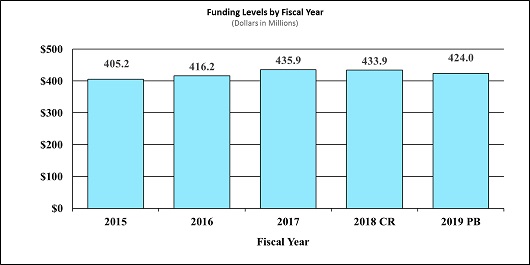
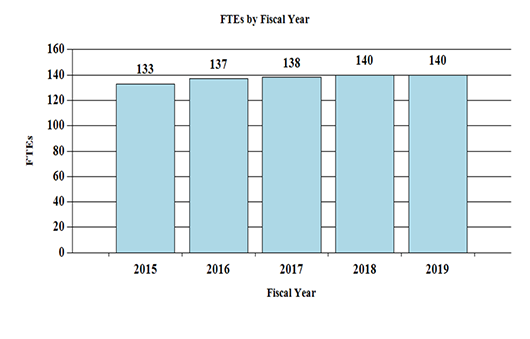
Fiscal Year 2019 Budget Graphs
History of Budget Authority and FTEs:
| PHS Act/Other Citation | U.S. Code Citation | 2018 Amount Authorized | FY 2018 Annualized CR | 2019 Amount Authorized | FY 2019 President's Budget | |
|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinite | $433,908,182 | Indefinite | $423,992,000 |
| National Institute on Deafness and Other Communication Disorders | Section 401(a) | 42§281 | Indefinite | Indefinite | ||
| Total, Budget Authority | $433,908,182 | $423,992,000 |
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation |
|---|---|---|---|---|
| 1 Budget Estimate to Congress includes mandatory financing. | ||||
| 2009 | $395,047,000 | $408,587,000 | $406,000,000 | $407,259,000 |
| Rescission | $0 | |||
| 2010 | $413,026,000 | $422,308,000 | $414,755,000 | $418,833,000 |
| Rescission | $0 | |||
| 2011 | $429,007,000 | $428,331,000 | $418,833,000 | |
| Rescission | $3,677,604 | |||
| 2012 | $426,043,000 | $426,043,000 | $410,482,000 | $417,061,000 |
| Rescission | $788,245 | |||
| 2013 | $417,297,000 | $418,562,000 | $416,272,755 | |
| Rescission | $832,546 | |||
| Sequestration | ($20,894,030) | |||
| 2014 | $422,936,000 | $420,125,000 | $404,049,000 | |
| Rescission | $0 | |||
| 2015 | $403,933,000 | $405,302,000 | ||
| Rescission | $0 | |||
| 2016 | $416,241,000 | $412,366,000 | $424,860,000 | $423,031,000 |
| Rescission | $0 | |||
| 20171 | $422,936,000 | $434,126,000 | $441,778,000 | $436,875,000 |
| Rescission | $0 | |||
| 2018 | $325,846,000 | $443,624,000 | $451,768,000 | $436,875,000 |
| Rescission | $2,966,818 | |||
| 2019 | $423,992,000 | |||
| FY 2017 Actual | FY 2018 Annualized CR | FY 2019 President's Budget | FY 2019 +/- FY 2018 | ||||
|---|---|---|---|---|---|---|---|
| BA | $435,904,000 | $433,908,182 | $423,992,000 | -$9,916,182 | |||
| FTE | 138 | 140 | 140 | 0 | |||
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director's Overview
An estimated 46 million people in the United States suffer some form of disordered communication.1 For those individuals, the basic components of communication (sensing, interpreting, and responding to people and things in our environment) can be challenging. The National Institute on Deafness and Other Communication Disorders (NIDCD) manages a broad intramural and extramural portfolio of both basic and clinical research focused on human communication research and their associated disorders in three program areas: hearing and balance; taste and smell; and voice, speech, and language.
NIDCD Research Advances: Extraordinary research opportunities have led to scientific breakthroughs in the study of genes, proteins, sensory and supporting cells, and molecular processes that directly affect our understanding of communication disorders. In the past year, NIDCD-supported research activities have led to advances in the following areas of communication science:
Supporting Basic Research into the Cause of Tinnitus2: NIDCD-supported scientists are conducting basic research to drive new understanding of disorders and have discovered an association between serotonin and tinnitus. Serotonin is a chemical in the body that acts as a neurotransmitter (helps carry signals between nerve cells). It is also responsible for maintaining mood balance. Tinnitus is commonly perceived as a constant ringing in the ears in the absence of sound. The scientists observed that neurons in the brain become hyperactive and hypersensitive when exposed to serotonin which may enhance the onset of tinnitus. Antidepressants that alleviate symptoms of depression and anxiety by increasing serotonin levels in the brain may worsen a person’s tinnitus. This finding may help physicians determine the appropriate treatment for an individual who has depression and also tinnitus or hearing loss.
Investing in Translational and Clinical Research to Improve Assistive Technology for Locked-In Syndrome3: Locked-in syndrome (LIS) is a state of near-total paralysis that happens as a result of stroke, end-stage neural degeneration, or neuromuscular disease, such as amyotrophic lateral sclerosis (Lou Gehrig’s disease). People with LIS have difficulty moving, or speaking and communicating with others. Brain-computer interface (BCI) technology uses sensors to measure electrical signals in the brain to run a computer program that can type words when individuals focus on letters on a virtual keyboard. These devices are effective in helping individuals with communication disorders, but users require considerable training and typing is very slow. NIDCD-supported scientists have significantly improved the performance of a BCI device by developing a program that combines data collected from electrical signals in the brain and from tracking eye movement. This hybrid system improved the accuracy and speed at which users could type words by glancing at a keyboard and using their thoughts. Future work will translate this technology from the lab into the clinic or home for individuals with LIS, potentially helping them communicate more easily and effectively with physicians, caretakers, and family.
Basic Science Uncovers Key Signals that Direct Stem Cell Identity in the Nose4: An NIDCD-funded study is exploring how stem cells in the nose are triggered to become either nerve cells or supporting cells. Unlike nerve cells in most of the brain, odor-sensing nerve cells in the adult nose are constantly being replaced by stem cells. When this process goes awry, you can lose your sense of smell. Approximately 30 percent of adults over the age of 70 have problems with their ability to smell.5 People without a good sense of smell detect flavors of food differently, and may not be able to detect spoiled food or gas leaks. This study used a sophisticated technique to track the genes expressed in single stem cells over time. By doing so, the researchers found some of the specific cellular signals that direct stem cells to become certain types of mature cells. This basic research may lead to treatments for people who lose their ability to smell.
NIDCD Plans, Priorities, and Challenges for the Future: NIDCD continues its support of new and competing Research Project Grants from investigators who have innovative ideas. NIDCD prioritizes its research investments to identify the most promising opportunities for human communication research, including:
- Improving Outcomes for Human Communication Disorders: NIDCD supports a variety of research to help individuals who have trouble communicating with others. For example, NIDCD supports research to help communication in individuals with a rare nervous system disorder called Rett syndrome. Previous research suggested that individuals with Rett syndrome are unable to communicate, but one NIDCD-funded project challenges that idea. Instead, the scientists observed that individuals with Rett syndrome use atypical communication methods (such as crying or “pointing” at something by looking at it instead of pointing with a finger). The scientists propose to use reinforcement-based systematic interventions to help individuals with Rett syndrome learn to communicate more effectively. NIDCD supported another study that tracks how and when individuals with age-related hearing loss use health services as compared to individuals without hearing loss. Individuals with hearing loss may misunderstand a doctor’s questions or instructions for medication use, and this can impact their health and quality of life. The results of this project will help us better understand how much hearing loss costs in both health and financial terms.
- Translating Basic Research into Clinical Tools: NIDCD supports projects that allow the findings of basic research to be developed into products that can be used to help patients. Through NIDCD support, scientists have developed tools to measure language development in people who speak both English and Spanish, and to measure impairment when the brain doesn’t process sound properly. They have also developed brain computer-interface software to enable communication for people with impairments who cannot communicate using speech or writing. Other projects include development of new drugs that might prevent noise-induced hearing loss, techniques that deliver antibiotics to the inner ear for treating middle-ear infection, treatments for ringing in the ears (tinnitus), and auditory implants to restore hearing for people who don’t benefit from a cochlear implant.
Program Descriptions and Accomplishments
Hearing and Balance Program: Loss of hearing or balance imposes a significant social and economic burden upon individuals, their families, and the communities in which they live. Millions of Americans experience a hearing or balance disorder at some point in their life, especially as young children or older adults. Common examples include middle-ear infections (otitis media), noise-induced hearing loss, tinnitus, age-related hearing loss, dizziness, and vertigo. Hearing and balance disorders also decrease quality of life, and cross all ethnic and socioeconomic lines. Approximately 36 million American adults report some degree of hearing loss6 and almost eight million adults report a chronic problem with balance.7 In addition, about 2 to 3 out of every 1,000 children in the United States are born with a detectable level of hearing loss in one or both ears that can affect their speech, language, social, and cognitive development.8 Accordingly, research projects within the NIDCD Hearing and Balance Program encompass over half of NIDCD’s portfolio. To study normal and disordered functions of the auditory and vestibular (balance) systems, NIDCD employs a wide range of research approaches such as molecular genetics, cellular biology, biomedical imaging, nanotechnology, psychoacoustics, and structural and functional biology. NIDCD supports research that will lead to improved treatments for, and prevention of, hearing and balance disorders.
On October 18, 2017, President Trump signed Public Law 115-71, the Early Hearing Detection and Intervention (EHDI) Act. The law amends the Public Health Service Act to reauthorize a federal program administered by the United States Department of Health and Human Services (HHS) until 2022. Under the law, NIDCD continues a basic and clinical research program on EHDI by leveraging a long-standing partnership with the Health Resources and Services Administration and the Centers for Disease Control and Prevention to coordinate a national program for the early detection, diagnosis, and intervention services for newborns and infants who are deaf and hard-of-hearing. As a result of the program, approximately 96 percent of United States newborns are screened for hearing loss before they are one month of age.9 Prior to 1993, under 10 percent of infants were screened for hearing loss, and about 50 percent of children with hearing loss went unidentified missing a critical period for language acquisition.10
NIDCD-supported research on EHDI includes studies on screening, assessment/diagnostics, and interventions. Examples of critical outcomes from this research include developing the technology to screen newborns for hearing loss, and demonstrating that children with hearing loss who get help early develop better speech and language skills than those who don’t. Continued EHDI research is needed to determine ideal, early intervention approaches for speech perception and production, and language (both spoken and signed) development, and to determine if early access to hearing health care changes health outcomes in children later in life. Further, research is needed to continue development and assessment of ongoing and novel methods for newborn hearing screening and to develop interventions and health care strategies to ensure that all children identified through a screening program receive appropriate diagnostic follow up in a timely fashion. Finally, NIDCD is committed to funding research to test the effectiveness of various types of early intervention strategies such as hearing aids and cochlear implants for infant and children who are deaf or hard-of-hearing.
NIDCD-supported scientists are investing in clinical research to improve communication outcomes in a large study aimed at identifying the best surgical approach and type of electrode for cochlear implantation.11 Cochlear implants can restore a sense of hearing in individuals who are deaf or severely hard-of-hearing, who do not benefit from hearing aids. The surgery involves inserting an electrode into the cochlea (the hearing organ), bypassing the damaged portions of the inner ear, and directly stimulating the auditory nerve. The cochlear implant turns sound waves into electrical signals that are sent to the brain. Sometimes residual hearing can be lost as a result of cochlear implantation. This large clinical study compared surgical approaches and electrode characteristics in nearly 200 patients to determine which variables have better rates of hearing preservation after surgery. Electrode arrays without a wire and surgical approaches (round window approach) that leave the cochlea intact were associated with the best rates of hearing preservation. Outcomes of this study will provide surgeons with evidence-based direction on the care and management of their patients who may benefit from a cochlear implant.
NIDCD-supported scientists have discovered how to generate “organoids” from stem cells that functionally mimic human inner ears cells.12 Replicating the function of human inner ear cells in the lab offers scientists the opportunity to study disorders of the inner ear. The scientists grew the stem cells using a novel 3-dimensional culturing method, where groups of cells are suspended in liquid rather than growing them flat on a dish. Researchers could then direct the stem cell to become inner ear organoids containing the sensory cells and supporting cells of the inner ear that are essential for hearing and balance. In addition, the organoids contained neurons, important to transmit signals from the ear to the brain, that formed connections with sensory cells. The scientists are currently using the human inner ear organoids to study how known genes that cause deafness interrupt normal development of the inner ear. They plan to start screening for drugs that could treat hearing and balance disorders using human inner ear organoids.
Program Portrait: Investing in Translational and Clinical Research to Improve Accessible and Affordable Hearing Health Care for Adults
Approximately 28.8 million adults13 in the United States could benefit from using hearing aids, yet only 1 in 4 has ever used one.14 Hearing loss often leads to isolation and has been associated with serious conditions such as depression, anxiety, low self-esteem, dementia, reduced mobility, and falls. The current Food and Drug Administration (FDA) regulations for selling hearing aids and the cost of hearing aids have been identified as barriers to accessible and affordable hearing health care. To this end, on August 18, 2017, President Trump signed Public Law 115-52, the FDA Reauthorization Act. The law directs the FDA to create a category of over-the-counter (OTC) hearing aids for adults with perceived mild-to-moderate hearing loss. Making OTC hearing aids available will make it easier for millions of American adults with perceived mild-to-moderate hearing loss to benefit from hearing aids. With the help of effective hearing devices, people can continue to engage with family and friends, maintain productivity at work, and are less likely to miss important instructions from health providers or safety officials.
NIDCD supported the consensus study that was a driving factor for the proposed legislation to mandate that FDA implement a new category of OTC hearing devices. Released June 2016 by the National Academies of Sciences, Engineering, and Medicine, “Hearing Health Care for Adults: Priorities for Improving Access and Affordability,” was cosponsored by the National Institute on Aging, four other federal agencies, and a nonprofit patient advocacy group. The study concluded that the diverse needs of adults with hearing loss were not being met. The report included other key recommendations to enhance consumers’ ability to find and use appropriate, affordable, and high-quality services, technologies, and support they need to manage hearing loss. The report also calls for more evidence-based information for the public and increased transparency of fee structures for devices and services, compatible technologies, and patients’ rights to their hearing health care records so they can make more informed decisions. In response to the report’s recommendations for further research, NIDCD continues to support adult hearing loss research that addresses multilevel, transdisciplinary population health interventions that target underlying social, economic, and environmental conditions to improve health outcomes.
NIDCD supports studies on technologies to help people hear better and on delivery models to get hearing loss interventions into the hands of the people who need them. For example, an NIDCD-sponsored randomized, double-blind, placebo controlled clinical trial was the first of its kind to compare the efficacy of two service-delivery models of hearing aids.15 Participants who selected pre-programmed hearing aids using an OTC delivery model reported a similar level of benefit as participants who purchased the same hearing aids through an audiologist following best practices. This study provides evidence-based support for an efficacious OTC hearing aid service delivery model that may increase accessibility and affordability of hearing aids for millions of Americans.
NIDCD continues to fund a broad range of hearing health research, including dozens of clinical and translational research projects to develop and test novel strategies to overcome barriers to care and to improve quality of life for the millions of American adults with hearing loss. NIDCD currently supports two funding opportunity announcements titled “Hearing Health Care for Adults: Improving Access and Affordability” to encourage evidence-based research that can lead to better ways to deliver better hearing health care and to make it more accessible and affordable.
Taste and Smell Program: Each year, more than 200,000 people visit a physician for chemosensory problems such as taste and smell disorders.16 Many more taste and smell disorders go unreported. NIDCD supports studies of the chemical senses known as taste, smell, and chemethesis (chemically provoked irritation) to enhance our understanding of how individuals communicate with their environment and how human chemosensory disorders can be diagnosed and treated. The regenerative capability of the olfactory (relating to our sense of smell) system declines with age, which negatively impacts olfactory function. Although the estimates of the prevalence of olfactory impairment vary, it is likely that more than one third of adults over the age of 70 have olfactory deficits.17 Since both taste and smell contribute to flavor perception, such olfactory deficits affect the flavor of foods and consequently food intake, diet and overall nutrition, and health status. NIDCD encourages further studies of this age-related decline in olfactory sensitivity including the development of better diagnostic tests to assess chemosensory loss and animal models to study why this decline occurs and how to prevent it.
Stem cells in our tongues allow us to replace lost taste cells throughout life. NIDCD-supported scientists are learning how taste stem cells give rise to the various types of cells found within a taste bud, including different taste receptor cells that detect sweet, sour, bitter, salty, or umami tasting substances.18 They isolated and grew taste stem cells into taste bud-like organoids in a culture dish. Next, they looked at which genes were turned on at each stage of taste bud generation. In early stages, multiple genes important for cell division were expressed, suggesting that active cell division occurs early in taste bud development. At later stages, the cells express a wide variety of taste-specific genes as well as a number of genes not previously implicated in taste cell development and function. Scientists are excited to have a new roster of genes important to establish taste cell identity and hope to learn how turning on an early gene influences turning on later genes. These new details about how the sense of taste develops may help scientists learn how to restore taste cells lost after chemotherapy for head and neck cancer, or how to influence taste to help people choose to eat more nutritious foods.
A recent NIDCD-funded project discovered that some of the same processes that cause inflammation and cell death in the nose can also promote regrowth of cells important for our sense of smell.19 As in the tongue, stem cells in our noses allow us to replace lost odor-detecting cells throughout life. Scientists want to better understand how these odor-detecting cells regenerate. Some people lose their sense of smell because long-term sinusitis and nasal cavity inflammation cause stem cells to stop replacing damaged odor-sensing cells. Interestingly, this mouse study found that one particular gene that is turned on during the process of inflammation actually helped to regenerate odor-sensitive tissue in the nose. When scientists blocked this gene in mice, the mice were less able to regrow odor-sensitive tissue after trauma. Scientists may now be able to use this new information about how odor-detecting cells regenerate to help people who have lost their sense of smell due to chemotherapy, chronic sinus infection, or other disorders of smell.
An NIDCD-funded scientist argues that the human sense of smell is much more sensitive than thought and that smell strongly influences what we think and how we behave, and evokes memories.20 A recent study showed that humans can potentially tell the difference between a huge number of odors and are in fact more sensitive than dogs and rodents for some odors. These findings challenge traditional beliefs about humans having a weak sense of smell compared to other creatures. Even though humans have fewer kinds of odor receptors in the nose, the olfactory bulb (the part of the brain that receives information from odor receptors) is of similar size relative to other mammals and has elaborate connections to higher areas of the human brain involved in emotion, memory, and behavior. NIDCD supports research to understand how humans perceive and process smells, and to explore the full capacity of the sense of smell.
NIDCD is dedicated to building a critical mass of scientists trained to treat problems with taste and smell. Current treatment options for taste and smell disorders are very limited and unable to help most people. As emphasized in the 2017-2021 NIDCD Strategic Plan, lack of treatment strategies is a critical gap in the chemical senses research field, especially given that taste and smell deficits have become increasingly common. NIDCD is committed to promoting clinical training in the chemical senses, and recently offered one-year administrative research supplements to active NIDCD-supported awards for medical students to gain research experience in basic, translational, or patient-oriented research in the chemical senses. NIDCD hopes that these awards will help establish a robust community of clinician-scientists with the expertise to develop preventative strategies and treatments for the many Americans who suffer from taste and smell disorders.
Voice, Speech, and Language Program: Disorders involving voice, speech, or language can have an overwhelming effect on an individual’s health and quality of life. These disorders affect people of all ages with or without hearing impairment, including children with autism, those who stutter, and adults with acquired communication disorders. Voice, speech, and language disorders also come at a significant cost. The societal burden of laryngeal disorders is estimated at $11 billion dollars annually due to work-related disability, lost productivity, and direct health care cost.21 Further, nearly eight percent of children ages 3-17 years have had a communication disorder during the past 12 months, according to data from the National Health Interview Survey, 2012.22 By the first grade, roughly five percent of children have noticeable speech disorders.23 In children, delayed speech and language acquisition or impairment are very often significant predictors of future academic, social, vocational, and adaptive outcomes.24
Many communication disorders, such as specific language impairment, childhood apraxia of speech, and stuttering, first become apparent when a child normally begins to acquire speech and language. Of children who experience stuttering, 25 percent will develop a persistent stuttering problem.25 NIDCD is committed to supporting research efforts to improve the identification of speech and language disorders such as stuttering in children and to improve treatments options. For example, scientists have been working to identify the possible genes responsible for stuttering that tend to run in families. NIDCD scientists have identified variants in four such genes that account for some cases of stuttering in many populations around the world, including the United States and Europe.26 All of these genes encode proteins that direct traffic within cells, ensuring that various cell components get to their proper location within the cell. Such deficits in cellular trafficking are a newly recognized cause of many neurological disorders. Researchers are now studying how this defect in cellular trafficking leads to specific deficits in speech fluency.
Researchers are also working to help speech-language pathologists determine which children are most likely to outgrow their stuttering and which children are at risk for continuing to stutter into adulthood. In addition, researchers are examining ways to identify groups of individuals who exhibit similar stuttering patterns and behaviors that may be associated with a common cause.
Scientists are using brain imaging tools such as positron emission tomography and functional magnetic resonance imaging scans to investigate brain activity in children who stutter. NIDCD-funded researchers are also using brain imaging to examine brain structure and functional changes that occur during childhood that differentiate children who continue to stutter from those who recover from stuttering. Brain imaging may be used in the future as a way to help treat people who stutter.
Other developmental disorders may also include communication problems, such as autism spectrum disorder (ASD), Fragile X, or cerebral palsy. One of the hallmarks of ASD is the diminished ability to communicate effectively—particularly in the expression and reception of language. ASD affects each person in different ways and can range from very mild to severe. According to the Centers for Disease Control and Prevention, about 1 in 68 children is diagnosed with an ASD.27 NIDCD has identified a number of research needs and opportunities related to children with autism, and in particular, among the 25-30 percent of children with ASD who remain functionally non-verbal beyond five years of age—a subset under-represented in research.28
To meet the needs of this vulnerable population, NIDCD supports Autism Centers of Excellence center grants some of which focus on the language abilities of children with ASD with the goal of helping them. Other funded projects aim to discover early behavioral and neural biomarkers to identify high-risk infants and children for ASD or different subgroups of children with ASD language impairments to develop targeted behavioral and/or pharmacological treatments to help prevent or reduce severity of the disorder. In a NIDCD-funded clinical trial, researchers will evaluate how parent characteristics influence outcomes for two different parent-mediated language interventions to determine the most effective strategies to reduce long-term communication difficulties for children with ASD. Another clinical research project tests the cost-effectiveness of a parent implemented treatment for siblings of children with ASD during infancy when their brains are still developing. Siblings who are not responsive to an initial low cost treatment can subsequently be referred to a more intense and expensive treatment. Current screening over-identifies siblings needing expensive treatment, and this study could have cost saving implications.
Stroke is a leading cause of adult disability in the United States.29 A significant proportion of stroke survivors have communication disorders, such as post-stroke difficulty in using language (aphasia) or difficulty in articulating words (dysarthria) as a result of the brain injury. Additionally, neurodegenerative disorders, such as Parkinson’s disease or amyotrophic lateral sclerosis, and injury can lead to impairments in planning and executing motor speech production such as in apraxia or dysarthria. These types of communication problems are a strong predictor of increased isolation and poor quality of life.30 NIDCD supports research to understand the neurological bases of voice, speech, and language impairments; the correlation of brain imaging data with prognosis; and the development of novel intervention strategies to improve outcomes.
For example, NIDCD supports research on primary progressive aphasia (PPA), a neurodegenerative disease associated with language decline. The gold standard for diagnosing PPA is via traditional structural magnetic resonance imaging (MRI) which assesses atrophy, or shrinkage, within the brain. Early stage disease does not display this atrophy, so making a diagnosis and starting early treatment is difficult. NIDCD-funded scientists provided proof of concept for a way to diagnose PPA earlier by using two brain imaging techniques—functional magnetic resonance imaging (fMRI) and traditional structural MRI.31 The researchers were able to measure reduced functional connectivity in the language networks of the brain in individuals that had pronounced language difficulties but no significant signs of brain atrophy. This advance may help physicians diagnose and treat individuals with PPA earlier, potentially allowing for better communication outcomes.
NIDCD also supports research to help understand the neurological basis of why some individuals with post-stroke chronic aphasia improve with speech treatment while others show little response. Significant advances in imaging technology has improved the understanding of the complex actions that take place in the part of the brain controlling human speech and has allowed for mapping of the functional connections of the brain that are responsible for speech control. In one NIDCD-sponsored study, researchers are using advanced imaging and a new analytical approach to understand what areas of the brain are important for treatment-induced speech recovery.32 They found that recovery is based on how well the language networks of the brain are able to restructure after stroke. These findings may help guide treatment decision making for individual with chronic aphasia from a stroke. The same imaging, analysis, and treatment strategies can also be applied to other conditions such as post-stroke motor rehabilitation, neurodegeneration, or traumatic brain injury.
Intramural Research Program (IRP): The NIDCD Intramural Research Program conducts basic and clinical research in human communication, with a primary focus on hearing and balance.
Enlargement of the vestibular aqueduct (EVA) has long been associated with hearing loss. Between 5 and 15 percent of children with sensorineural hearing loss (hearing loss caused by damage to sensory cells inside the cochlea) have EVA.33 A new study conducted by NIDCD intramural scientists using a mouse model finally reveals the root cause of how this structure becomes enlarged.34 For the first time, researchers gained insight into the functional, molecular, and cellular architecture of the endolymphatic sac, a balloon shaped structure attached to the cochlea, and identified the components of the developmental pathway that is disrupted in EVA. Identifying and understanding these cells could lead to new approaches to preventing and treating hearing loss associated with EVA and similar disorders.
In another study, NIDCD intramural scientists discovered a gene mutation that causes sensorineural hearing loss.35 The gene is responsible for normal immune responses in the cochlea by regulating a secreted inflammatory factor called IL-1β. When individuals with the mutated gene and hearing loss were treated by blocking the activity of IL-1β, the hearing loss was reversed in children and improved in a young adult. By understanding the underlying pathway, scientists hope to treat a variety of hearing loss disorders, such as sudden sensorineural hearing loss and Meniere’s disease that are caused by pathogens or processes that stimulate a person’s own immune response within the cochlea.
NIDCD intramural scientists use animal models to investigate the molecules and factors important for normal inner ear development. These researchers demonstrated that a protein called Emx2 is critical to how specialized cells that are important for maintaining hearing and balance are positioned in the inner ear.36 Emx2 is a transcription factor, a type of protein that plays a role in regulating other genes. The research offers new insight into how specialized sensory hair cells develop and function, providing opportunities for scientists to explore novel ways to treat hearing loss, balance disorders, and deafness.
The NIDCD Intramural Research Program also conducts research on head and neck cancer, as the disease as well as the treatment can impair human communication. Some individuals become resistant to effective cancer treatment over time. NIDCD clinical scientists are working to find ways to prevent cancer treatment resistance by manipulating an individual’s immune system. For example, using a mouse model, NIDCD intramural scientists identified a type of immune cell in the tumor environment that interfered with cancer treatments for head and neck cancer.37,38 By decreasing those immune cells’ function, researchers were able to make an existing cancer treatment more effective. Next, researchers hope to translate this finding from the bench into the clinic to improve head and neck cancer treatment outcomes and prevent or reduce human communication impairments.
Program Portrait: Investing in Translation and Clinical Research to Improve Health - Rare Diseases
What scientists learn from studying rare diseases can also help them understand more common conditions. At NIDCD, studying how and why inherited mutations in rare diseases cause hearing loss is helping our scientists understand the complex interactions of cells in the inner ear. They can use what they learn about how things go wrong in rare diseases to develop ways to prevent and treat hearing loss in everyone. NIDCD’s Intramural Research Program is working to understand several rare diseases that affect how we hear.
In individuals with Enlarged Vestibular Aqueduct (EVA), canals inside the skull that house the organs that detect hearing and balance swell, and become enlarged. EVA also causes hearing loss. NIDCD intramural scientists studied how the canals become enlarged in a mouse model of EVA. By identifying which genes are mutated and what goes wrong in the EVA mouse ear, they identified genes critical for the developing inner ear’s ability to regulate fluids. In both mice and humans, inherited mutations in these genes cause problems regulating fluids in the inner ear, and the vestibular aqueduct swells, causing EVA.39 They now hope to figure out how mutations in these genes also cause hearing loss.
Individuals with the mutations that cause Usher syndrome are born deaf and gradually lose their vision. NIDCD intramural scientists corrected the mutation that causes a particularly severe form of Usher syndrome in a mouse model of the disease.40 By injecting a harmless virus that also carried a corrected version of the mutant gene, they were able to restore the mice’s ability to hear and balance. Now they hope to use what they’ve learned to restore hearing in individuals with Usher syndrome.
Niemann-Pick Type C Disease is a neurodegenerative disease caused by problems with nerve cells’ ability to manage their cellular waste products. Nerve cells need to get rid of cholesterol in order to work properly, but the Niemann-Pick type C mutation causes cholesterol to build up and kill the cells. Individuals with Niemann-Pick Type C mutations also have hearing loss. NIDCD intramural scientists participated in a clinical trial testing a drug to treat individuals with Neimann-Pick Type C disease, monitoring their hearing and providing hearing aids as needed.41 Although the trial determined that the drug slowed progression of this deadly disease, it unfortunately also causes hearing loss. Now NIDCD intramural scientists are working to understand why and how the drug causes hearing loss.
Along with a host of other problems, Smith-Magenis syndrome can cause hearing loss and sensitivity to environmental sounds. NIDCD intramural scientists are the first to describe changes in hearing over time in a subgroup of these individuals.42 Their report helps parents and others educating and caring for individuals with Smith-Magenis syndrome understand what to expect. The scientists recommend monitoring hearing as part of clinical care to identify changes in hearing and provide interventions as needed.
Alstrom syndrome is a rare disease caused by problems with how cilia work. Cilia are hair-like extensions on the tips of cells, and are responsible for moving things in the body, such as air in the lungs and in the nose, or fluid in the kidneys and in the inner ears. Individuals with Alstrom syndrome develop hearing loss after birth, and NIDCD intramural scientists documented when hearing loss was first observed in these individuals, and how hearing loss progressed over time.43 Their report helps these individuals and their healthcare providers understand what to expect with their hearing loss, and also how best to treat their hearing loss.
Research Management and Support (RMS) Program: NIDCD RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS functions also include strategic planning, coordination, and evaluation of the Institute’s programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. The Institute currently supports approximately 1,300 research grants, training awards, and R&D contracts.
NIDCD continues to be a good steward of its resources in supporting biomedical research on human communication disorders and in supporting the administrative management of research through strategic planning processes. The Institute completed its implementation of the NIDCD Administrative Strategic Plan (ASP) which facilitated effective and efficient administrative management services in support of NIDCD’s mission to advance biomedical research discoveries. The ASP focused on four areas to improve administrative services: Human Resources Engagement, Human Resources Services, Information Technology, and Employee Training and Support. In addition to improving administrative services, the ASP provided important opportunities to develop leadership and teambuilding competencies among NIDCD employees. The ASP required thoughtful input and dedication from staff across NIDCD and was developed through staff feedback from the Federal Employees Viewpoint Survey and from NIDCD leadership.
NIDCD developed and supports a public education campaign called “It’s a Noisy Planet. Protect Their Hearing®.” The campaign is designed to increase awareness among parents of children ages 8 to 12 about the causes and ways to prevent noise-induced hearing loss (NIHL). In 2017, NIDCD expanded training information for health professionals and educators to deliver NIHL prevention messaging into schools across the country for Grades 2 through 6. The Noisy Planet website now offers a virtual Teacher Toolkit44 that includes a downloadable presentation with accompanying talking points, video demonstrations, and instructions for hands-on activities to explain what sound is, how sound travels through the ear, how loud sound can damage hearing over time, and how to protect your hearing. Parents and other adults can encourage children to adopt healthy hearing habits as they develop listening, leisure, and working habits.
| OFFICE/DIVISION | FY 2017 Final | FY 2018 Annualized CR | FY 2019 President's Budget | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Fiscal Year | Average GS Grade | ||||||||
| Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||||||||
| Division of Extramural Activities | |||||||||
| Direct: | 19 | - | 19 | 19 | - | 19 | 19 | - | 19 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 19 | - | 19 | 19 | - | 19 | 19 | - | 19 |
| Division of Intramural Research Program | |||||||||
| Direct: | 61 | 1 | 62 | 62 | 1 | 63 | 62 | 1 | 63 |
| Reimbursable: | 4 | - | 4 | 4 | - | 4 | 4 | - | 4 |
| Total: | 65 | 1 | 66 | 66 | 1 | 67 | 66 | 1 | 67 |
| Division of Scientific Programs | |||||||||
| Direct: | 16 | - | 16 | 17 | - | 17 | 17 | - | 17 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 16 | - | 16 | 17 | - | 17 | 17 | - | 17 |
| Office of Administration | |||||||||
| Direct: | 34 | - | 34 | 34 | - | 34 | 34 | - | 34 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 34 | - | 34 | 34 | - | 34 | 34 | - | 34 |
| Office of the Director | |||||||||
| Direct: | 3 | - | 3 | 3 | - | 3 | 3 | - | 3 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 3 | - | 3 | 3 | - | 3 | 3 | - | 3 |
| Total: | 137 | 1 | 138 | 139 | 1 | 140 | 139 | 1 | 140 |
| FTEs supported by funds from Cooperative Research and Development Agreements. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2015 | 12.4 | ||||||||
| 2016 | 12.1 | ||||||||
| 2017 | 12.1 | ||||||||
| 2018 | 12.1 | ||||||||
| 2019 | 12.1 | ||||||||
| GRADE | FY 2017 Final | FY 2018 Annualized CR | FY 2019 President's Budget |
|---|---|---|---|
| 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | 187,000 | 191,282 | 192,200 |
| GM/GS-15 | 20 | 21 | 21 |
| GM/GS-14 | 17 | 17 | 17 |
| GM/GS-13 | 22 | 22 | 22 |
| GS-12 | 19 | 20 | 20 |
| GS-11 | 7 | 7 | 7 |
| GS-10 | 7 | 7 | 7 |
| GS-9 | 5 | 5 | 5 |
| GS-8 | 3 | 3 | 3 |
| GS-7 | 1 | 1 | 1 |
| GS-6 | 0 | 0 | 0 |
| GS-5 | 0 | 0 | 0 |
| GS-4 | 1 | 1 | 1 |
| GS-3 | 1 | 1 | 1 |
| GS-2 | 2 | 2 | 2 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 105 | 107 | 107 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): | 0 | 0 | 0 |
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 0 | 0 | 0 |
| Senior Grade | 1 | 1 | 1 |
| Full Grade | 0 | 0 | 0 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 1 | 1 | 1 |
| Ungraded | 44 | 44 | 44 |
| Total permanent positions | 103 | 105 | 105 |
| Total positions, end of year | 150 | 152 | 152 |
| Total full-time equivalent (FTE) employment, end of year | 138 | 140 | 140 |
| Average ES salary | 187,000 | 191,282 | 192,200 |
| Average GM/GS grade | 12.1 | 12.1 | 12.1 |
| Average GM/GS salary | 111,834 | 113,959 | 114,506 |
- /about/strategic-plan/2017-2021
- https://www.ncbi.nlm.nih.gov/pubmed/28834748
- https://www.ncbi.nlm.nih.gov/pubmed/28585523
- https://www.ncbi.nlm.nih.gov/pubmed/28506465
- NIDCD Epidemiology and Statistics Branch calculation
- Based on NCHS/NHIS data for 2007.
- Based on prevalence data from 1994–95 Disability Supplement to the NHIS and current US population estimates.
- http://www.ncbi.nlm.nih.gov/pubmed/20203554, http://www.ncbi.nlm.nih.gov/pubmed/12784222
- /health/your-babys-hearing-screening-and-next-steps
- NIH Consensus Statement. Volume 11, Number 1, March 1-3, 1993.
- https://www.ncbi.nlm.nih.gov/pubmed/28643327
- https://www.ncbi.nlm.nih.gov/pubmed/28459451
- /health/statistics/quick-statistics-hearing
- Based on calculations by NIDCD Epidemiology and Statistics Branch using data from NHIS and NHANES
- https://www.ncbi.nlm.nih.gov/pubmed/28252160
- /health/statistics/quick-statistics-taste-smell
- NIDCD Epidemiology and Statistics Branch calculation
- https://www.ncbi.nlm.nih.gov/pubmed/28638111
- https://www.ncbi.nlm.nih.gov/pubmed/28696292
- https://www.ncbi.nlm.nih.gov/pubmed/28495701
- https://www.ncbi.nlm.nih.gov/pubmed/22549455, https://www.ncbi.nlm.nih.gov/pubmed/22544473
- https://www.cdc.gov/nchs/products/databriefs/db205.htm
- http://www.ahrq.gov/downloads/pub/prevent/pdfser/speechsyn.pdf (1.3 KB PDF)
- https://www.ncbi.nlm.nih.gov/pubmed/18695010, https://www.ncbi.nlm.nih.gov/pubmed/17883441, https://www.ncbi.nlm.nih.gov/pubmed/15679523
- /health/stuttering
- https://www.ncbi.nlm.nih.gov/pubmed/20147709, http://www.cell.com/ajhg/fulltext/S0002-9297(15)00409-7
- Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010. CDC, March 28, 2014. http://www.cdc.gov/mmwr/preview/mmwrhtml/ss6302a1.htm
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3869868/
- https://www.ncbi.nlm.nih.gov/pubmed/21160056
- https://www.ncbi.nlm.nih.gov/pubmed/11776713
- https://www.ncbi.nlm.nih.gov/pubmed/28288010
- https://www.ncbi.nlm.nih.gov/pubmed/28628946
- /health/enlarged-vestibular-aqueducts-and-childhood-hearing-loss
- https://www.ncbi.nlm.nih.gov/pubmed/28994389
- https://www.ncbi.nlm.nih.gov/pubmed/28847925
- https://www.ncbi.nlm.nih.gov/pubmed/28266911
- https://www.ncbi.nlm.nih.gov/pubmed/28364000
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5593525/
- https://www.ncbi.nlm.nih.gov/pubmed/28994389
- https://www.ncbi.nlm.nih.gov/pubmed/28254438
- https://www.ncbi.nlm.nih.gov/pubmed/28803710
- https://www.ncbi.nlm.nih.gov/pubmed/28384694
- https://www.ncbi.nlm.nih.gov/pubmed/28573831
- https://www.nidcd.nih.gov/health/teacher-toolkit-for-preventing-nihl
* Note: PDF files require a viewer such as the free Adobe Reader.

