Researchers believe that stuttering—a potentially lifelong and debilitating speech disorder—stems from problems with the circuits in the brain that control speech, but precisely how and where in the brain these problems occur is unknown. Using a mouse model of stuttering, scientists report that a loss of cells called astrocytes in the brain is associated with stuttering. The mice had been engineered with a human gene mutation previously linked to stuttering. The study, which appeared online in the Proceedings of the National Academy of Sciences in August, offers insights into the neurological deficits associated with stuttering.
The loss of astrocytes, a type of supporting cell, was most prominent in the corpus callosum, a part of the brain that bridges the two hemispheres. Previous imaging studies have identified differences in the brains of people with persistent developmental stuttering compared to those who do not stutter. Some of these studies have revealed structural and functional problems in the same brain region as the new mouse study.
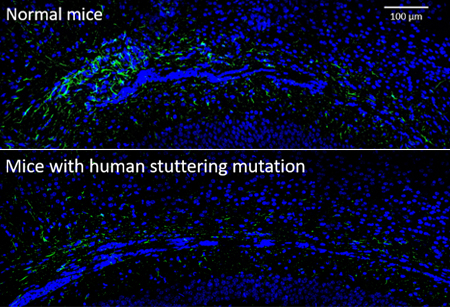
In the corpus callosum (the area of the brain that enables the left and right hemispheres to communicate) of mice engineered with a human stuttering mutation (lower panel), there are fewer astrocytes, shown in green, than in normal mice (upper panel).
Source: Tae-Un Han, Ph.D., NIDCD/NIH
The study was led by Dennis Drayna, Ph.D., of the Section on Genetics of Communication Disorders at the National Institute on Deafness and Other Communication Disorders (NIDCD), part of the National Institutes of Health. Researchers at the Washington University School of Medicine in St. Louis and from two additional components of NIH—the National Institute of Biomedical Imaging and Bioengineering and the National Institute of Mental Health—collaborated on the research.
“The identification of genetic, molecular, and cellular changes that underlie stuttering has led us to understand persistent stuttering as a brain disorder,” said Andrew Griffith, M.D., Ph.D., NIDCD scientific director. “Perhaps even more importantly, pinpointing the brain region and cells that are involved opens opportunities for novel interventions for stuttering—and possibly other speech disorders.”
Stuttering is characterized by pauses and repeated or prolonged sounds, syllables, or words that disrupt the normal flow of speech. People who stutter know what they want to say, but they have trouble saying it. The condition is most commonly seen in young children, who typically outgrow the problem. However, for 1 in 4 children who experience early stuttering, the condition persists as a lifelong communication problem. It is estimated that as many as 1% of adults in the United States are affected by stuttering.
“The brain imaging studies of people who stutter are important, but those results can only take us so far,” said Drayna. One challenge, he said, is that the imaging studies cannot decipher if the differences contribute to stuttering or are an effect of stuttering.
“By taking a genetic approach, we have been able to begin deciphering the neuropathology of stuttering, first at the molecular level by identifying genetic mutations, and now at the cellular level,” added Drayna.
Earlier research by Drayna and colleagues identified several genes associated with stuttering. In this study, the researchers set out to identify changes in the brain brought on by the mutations in a gene called GNPTAB, one of the genes previously linked to stuttering. The scientists engineered this human stuttering mutation into the mice. The mice with the GNPTAB mutation had long pauses in their streams of vocalizations, somewhat similar to those found in the speech of people with the same mutation. Like people who stutter, the mice were normal in all other ways, reinforcing earlier research that suggests that the mice can serve as a valid animal model for important features of this disorder.
The investigators next examined brain tissue from the mice and found a decrease in astrocytes, but not other cell types, in the animals with the genetic mutation compared to the mice without the mutation. Astrocytes play a critical role in supporting nerve cells by carrying out a wide range of functions, such as supplying nerve cells with oxygen and nutrients and providing structural support.
The loss of astrocytes was more pronounced in the corpus callosum of the mutant mice. In addition, using advanced magnetic resonance imaging (MRI) methods, the researchers detected reduced local volume of the corpus callosum in the mutant mice despite normal diffusion tensor MRI values, providing further support for the finding of a defect in this brain region.
Containing as many as 200 million nerve fibers, the corpus callosum enables communication between the brain’s left and right hemispheres, helping to integrate signals for processes that involve both hemispheres, such as physical coordination and use of language.
Follow-up experiments in which the GNPTAB human stuttering mutation was introduced into individual brain cell types—rather than the entire mouse—confirmed that the vocalization defect is specific to astrocytes. The mice did not have the stuttering-like vocalizations when the mutation was engineered into other types of brain cells.
All of the stuttering genes that have been identified over the past decade are involved in intracellular trafficking, the process that cells use to move proteins and other components to their correct locations within the cell. Defects in cellular trafficking have been linked to other neurological disorders, such as amyotrophic lateral sclerosis (ALS), Parkinson’s disease, and Alzheimer’s disease, suggesting that certain nerve cell pathways are particularly sensitive to impairment of this process. The research does not indicate, however, that persistent stuttering is an early indicator of these other disorders.
If future research confirms that stuttering in people with GNPTAB mutations derives from a loss of astrocytes in the brain, the findings could open the door to new therapeutic strategies for some people with persistent developmental stuttering by targeting associated molecular pathways and cells.
This press release describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is foundational to advancing new and better ways to prevent, diagnose, and treat disease. Science is an unpredictable and incremental process; each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without the knowledge gained through basic research.
This research was supported by the Intramural Research Program of the NIH, by the NIDCD (Z1A-000046-18), the National Institute of Mental Health Rodent Behavioral Core, and the National Heart, Lung, and Blood Institute Animal MRI Core.
About the National Institute on Deafness and Other Communication Disorders (NIDCD): The NIDCD supports and conducts research and research training on the normal and disordered processes of hearing, balance, taste, smell, voice, speech, and language and provides health information, based upon scientific discovery, to the public.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 institutes and centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.

